Allyl Syringate
Abstract
1. Introduction
2. Results and Discussion
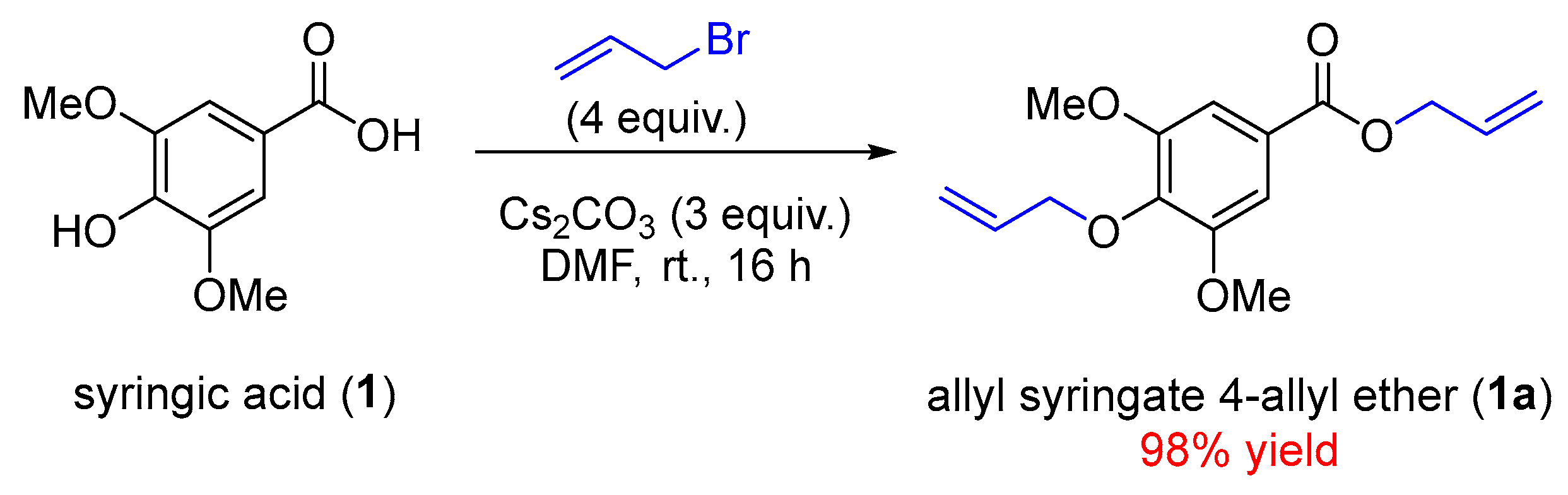
2.1. The Synthesis of Allyl Syringate (1c) and Related Derivatives (1a–1b)
2.2. Characterization of the Allylated Compounds (1a–1c)
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthesis of 4-(Allyloxy)-3,5-dimethoxybenzoate or Allyl Syringate 4-Allyl Ether (1a)
3.3. Synthesis of 4-(Allyloxy)-3,5-dimethoxybenzoic Acid or Syringic Acid 4-Allyl Ether (1b)
3.4. Synthesis of Allyl Syringate (1c)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| rt | Room temperature |
| equiv. | Equivalent |
| h | Hour |
| min | Minute |
| N | Normal |
References
- Raut, N.; Wicks, S.M.; Lawal, T.O.; Mahady, G.B. Epigenetic regulation of bone remodeling by natural compounds. Pharmacol. Res. 2019, 147, 104350. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Shimsa, S.; Mondal, S.; Mini, S. Syringic acid: A promising phenolic phytochemical with extensive therapeutic applications. R D Funct. Food Prod. 2024, 1, 1–14. [Google Scholar]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Guzmán, L.; Venturini, W.; Zapata-Gomez, F.; Duarte, Y.; Camargo-Ayala, L.; Echeverría, C.; Echeverría, J. O-Alkyl derivatives of ferulic and syringic acid as lipophilic antioxidants: Effect of the length of the alkyl chain on the improvement of the thermo-oxidative stability of sunflower oil. RSC Adv. 2024, 14, 22513–22524. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Khatkar, A.; Dhiman, P. Computational analysis and synthesis of syringic acid derivatives as xanthine oxidase inhibitors. Med. Chem. 2020, 16, 643–653. [Google Scholar] [CrossRef]
- Orabi, K.Y.; Abaza, M.S.; El Sayed, K.A.; Elnagar, A.Y.; Al-Attiyah, R.; Guleri, R.P. Selective growth inhibition of human malignant melanoma cells by syringic acid-derived proteasome inhibitors. Cancer Cell Int. 2013, 13, 82. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef]
- Federsel, H.J. What enables and blocks synthetic chemistry methods in becoming industrially significant? Cell Rep. Phys. Sci. 2023, 4, 101493. [Google Scholar] [CrossRef]
- Abdul Fattah, T.; Saeed, A. Applications of Keck allylation in the synthesis of natural products. New J. Chem. 2017, 41, 14804–14821. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Sanmartin, C.; Sharma, A.K.; Plano, D. From natural sources to synthetic derivatives: The allyl motif as a powerful tool for fragment-based design in cancer treatment. J. Med. Chem. 2023, 66, 3703–3731. [Google Scholar] [CrossRef]
- Diner, C.; Szabó, K.J. Recent advances in the preparation and application of allylboron species in organic synthesis. J. Am. Chem. Soc. 2017, 139, 2–14. [Google Scholar] [CrossRef]
- Dutta, S.; Bhattacharya, T.; Werz, D.B.; Maiti, D. Transition-metal-catalyzed CÀH allylation reactions. Chem 2021, 7, 555–605. [Google Scholar] [CrossRef]
- Lee, J.H. Use of the Hosomi-Sakurai allylation in natural product total synthesis. Tetrahedron 2020, 76, 131351. [Google Scholar] [CrossRef]
- Pang, M.; Hao, W.; Li, X.; Zhang, C.; Morsali, A.; Ramazani, A.; Zhang, G. Efficient allylation of dihalides: A versatile approach to C/N/O-functionalized derivatives. Chin. J. Chem. 2025, 43, 2005–2014. [Google Scholar] [CrossRef]
- Ravichandiran, V.; Jana, A. Recent development of allyl–allyl cross-coupling and its application in natural product synthesis. Org. Chem. Front. 2023, 10, 267–281. [Google Scholar] [CrossRef]
- Zeng, Q.; Yin, X.; Wang, Y. Progress on transition metal-catalyzed allylation of activated vinylcyclopropanes. Tetrahedron Lett. 2023, 123, 154536. [Google Scholar] [CrossRef]
- Ahmadi, Y. Allylation of aldehydes with various allylation agents. Results Chem. 2024, 11, 101847. [Google Scholar] [CrossRef]
- Butt, N.A.; Zhang, W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015, 44, 7929–7967. [Google Scholar] [CrossRef]
- Ghorai, D.; Cristòfol, À.; Kleij, A.W. Nickel-catalyzed allylic substitution reactions: An evolving alternative. Eur. J. Inorg. Chem. 2022, 2022, e202100820. [Google Scholar] [CrossRef]
- Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E.; Guiry, P.J.; Moberg, C.; Bäckvall, J.-E.; Pfaltz, A.; Pericàs, M.A.; et al. Recent advances in enantioselective Pd-catalyzed allylic substitution: From design to applications. Chem. Rev. 2021, 121, 4373–4505. [Google Scholar] [CrossRef]
- Ospina, F.; Schülke, K.H.; Hammer, S.C. Biocatalytic alkylation chemistry: Building molecular complexity with high selectivity. ChemPlusChem 2022, 87, e202100454. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Wang, Y.; Liu, Y.; Zhu, Y.; You, H.; Chen, F.E. Copper-catalyzed asymmetric allylic substitution of racemic/meso substrates. Chem. Sci. 2024, 15, 8280–8294. [Google Scholar] [CrossRef]
- Danraka, A.; Abdulmujeeb, A. The significance of synthetic chemistry in advancing pharmaceutical research and development in Nigeria: A call to action. Niger. J. Pharm. 2023, 57, 758–764. [Google Scholar] [CrossRef]
- Bielowicz, B. Waste as a source of critical raw materials—A new approach in the context of energy transition. Energies 2025, 18, 2101. [Google Scholar] [CrossRef]
- Ahmadli, D.; Müller, S.; Xie, Y.; Smejkal, T.; Jaeckh, S.; Iosub, A.V.; Williams, S.R.; Ritter, T. Standardized approach for diversification of complex small molecules via aryl thianthrenium salts. J. Am. Chem. Soc. 2025, 147, 4268–4283. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; An, M.; Jeon, M.; Kim, J.; Kang, S.M.; Choi, I. Sustainable approaches for the protection and deprotection of functional groups. Chem. Eur. J. 2025, 31, e202501387. [Google Scholar] [CrossRef]
- Yus, M.; González-Gómez, J.C.; Foubelo, F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 2011, 111, 7774–7854. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, R.; Liu, W.H. Recent advances of the Grignard-type reactions without involving organohalides. Tetrahedron Chem. 2024, 9, 100069. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, G.S.; Kapur, M. Oxazolinyl-assisted Ru(II)-catalyzed C–H allylation with allyl alcohols and synthesis of 4-methyleneisochroman-1-ones. J. Org. Chem. 2019, 84, 12881–12892. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry: Theory and Practice, 1st ed.; Anastas, P.T., Warner, J.C., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 29–56. [Google Scholar]
- Guoqing, L.; Zhan, L.; Xiuhua, F. A new convenient synthetic procedure for 4-allyl-2, 6-dimethoxyphenol. Synth. Commun. 1996, 26, 2569–2572. [Google Scholar] [CrossRef]
- Pandey, S.K.; Ramana, C. Total synthesis of (±)-sacidumlignan D. J. Org. Chem. 2011, 76, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhu, S.; Liu, Y.; Zhao, J.; Zhang, S.; Zhong, C. Quantitative methylation of lignin monomers using tetrabutylammonium hydroxide and MeI and applications in organic synthesis. ACS Omega 2023, 8, 7057–7062. [Google Scholar] [CrossRef]
- Wolrab, D.; Kohout, M.; Boras, M.; Lindner, W. Strong cation exchange-type chiral stationary phase for enantioseparation of chiral amines in subcritical fluid chromatography. J. Chromatogr. A 2013, 1289, 94–104. [Google Scholar] [CrossRef]
- Nowatschin, V.; Näther, C.; Lüning, U. Synthesis and crystal structure of allyl 7-(diethylamino)-2-oxo-2H-chromene-3-carboxylate. Struct. Rep. 2021, 77, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Harnoy, A.J.; Slor, G.; Tirosh, E.; Amir, R.J. The effect of photoisomerization on the enzymatic hydrolysis of polymeric micelles bearing photo-responsive azobenzene groups at their cores. Org. Biomol. Chem. 2016, 14, 5813–5819. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thimpa, N.; Poprom, S.; Songpao, L.; Chotsaeng, N. Allyl Syringate. Molbank 2025, 2025, M2060. https://doi.org/10.3390/M2060
Thimpa N, Poprom S, Songpao L, Chotsaeng N. Allyl Syringate. Molbank. 2025; 2025(3):M2060. https://doi.org/10.3390/M2060
Chicago/Turabian StyleThimpa, Naruedech, Suriyaphong Poprom, Laksakarn Songpao, and Nawasit Chotsaeng. 2025. "Allyl Syringate" Molbank 2025, no. 3: M2060. https://doi.org/10.3390/M2060
APA StyleThimpa, N., Poprom, S., Songpao, L., & Chotsaeng, N. (2025). Allyl Syringate. Molbank, 2025(3), M2060. https://doi.org/10.3390/M2060






