Two Cocrystals of Phenazine with Different Phenylboronic Acids
Abstract
1. Introduction
2. Results and Discussion
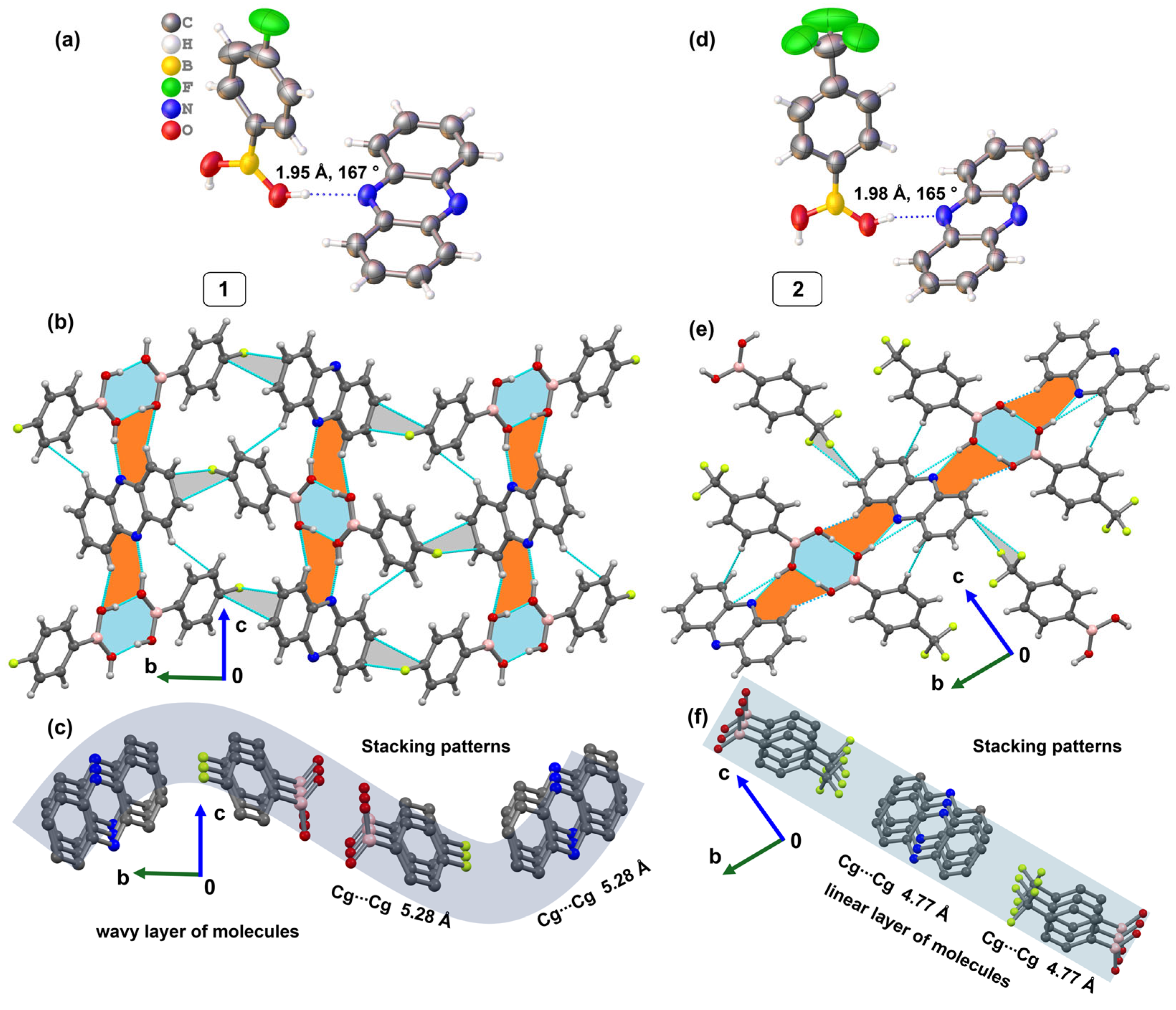
2.1. Crystal Structure Determination of Cocrystals 1 and 2
2.2. Supramolecular Features of Cocrystals 1 and 2
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hall, D.G. Boronic acid catalysis. Chem. Soc. Rev. 2019, 48, 3475–3496. [Google Scholar] [CrossRef] [PubMed]
- António, J.P.M.; Russo, R.; Carvalho, C.P.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids as building blocks for the construction of Therapeutically useful bioconjugates. Chem. Soc. Rev. 2019, 48, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- António, J.P.M.; Roque, I.L.; Santos, F.M.; Gois, P.M. Designing functional and responsive molecules with boronic acids. Acc. Chem. Res. 2025, 58, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Alvarado, G.; MacGillivray, L.R. Opportunities Using Boron to Direct Reactivity in the Organic Solid State. Synlett 2021, 32, 655–662. [Google Scholar]
- Bhandary, S.; Belis, M.; Kaczmarek, A.M.; Van Hecke, K. Photomechanical Motions in Organoboron-Based Phosphorescent Molecular Crystals Driven by a Crystal-State [2 + 2] Cycloaddition Reaction. J. Am. Chem. Soc. 2022, 144, 22051–22058. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Wang, C.; Wang, J.; Liu, F.; Xie, Y.; Zhang, Y.-Z.; Li, J.-R.; Li, Q.; Li, Z. Abnormal room temperature phosphorescence of purely organic boron-containing compounds: The relationship between the emissive behavior and the molecular packing, and the potential related applications. Chem. Sci. 2017, 8, 8336–8344. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, I.; Kervyn, S.; Rossignon, A.; De Leo, F.; Wouters, J.; Bruylants, G.; Bonifazi, D. Versatile self-adapting boronic acids for H-bond recognition: From discrete to polymeric supramolecules. J. Am. Chem. Soc. 2017, 139, 2710–2727. [Google Scholar] [CrossRef] [PubMed]
- SeethaLekshmi, S.; Varughese, S.; Giri, L.; Pedireddi, V.R. Molecular Complexes of 4-Halophenylboronic Acids: A Systematic Exploration of Isostructurality and Structural Landscape. Cryst. Growth Des. 2014, 14, 4143–4154. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. CCDC2009236: Experimental Crystal Structure Determination. Cambridge Structural Database (CSD) 2020. [Google Scholar] [CrossRef]
- Dostál, L.; Jambor, R.; Růžička, A.; Jirásko, R.; Lyčka, A.; Beckmann, J.; Ketkov, S. From Stiba-and Bismaheteroboroxines to N,C,N-Chelated Diorganoantimony(III) and Bismuth(III) Cations—An Unexpected Case of Aryl Group Migration. Inorg. Chem. 2015, 54, 6010–6019. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, S.; Shukla, R.; Van Hecke, K. Effect of chemical substitution on the construction of boroxine-based supramolecular crystalline polymers featuring B← N dative bonds. CrystEngComm 2022, 24, 1695–1699. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, version 1.171.40.81a; Rigaku Corporation: Oxford, UK, 2020.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]

| Identification Code | 1 | 2 |
|---|---|---|
| Empirical formula | C24H20B2F2N2O4 | C26H20B2F6N2O4 |
| CCDC no. | 2455586 | 2455587 |
| Formula weight/gmol−1 | 460.04 | 560.06 |
| Temperature/K | 293(2) | 293(2) |
| Crystal system | monoclinic | triclinic |
| Space group | P21/n | P-1 |
| a/Å | 5.2892(3) | 4.7792(3) |
| b/Å | 18.7736(11) | 11.4844(5) |
| c/Å | 11.5458(6) | 12.5192(7) |
| α/° | 90 | 94.233(4) |
| β/° | 102.544(6) | 99.337(5) |
| γ/° | 90 | 101.535(5) |
| Volume/Å3 | 1119.10(11) | 660.31(7) |
| Z | 2 | 1 |
| ρcalcg/cm3 | 1.365 | 1.408 |
| μ/mm−1 | 0.860 | 1.058 |
| F(000) | 476.0 | 286.0 |
| Crystal size/mm3 | 0.42 × 0.07 × 0.03 | 0.19 × 0.09 × 0.07 |
| Radiation | Cu Kα (λ = 1.54184 Å) | Cu Kα (λ = 1.54184 Å) |
| 2Θ range for data collection/° | 9.152 to 147.804 | 7.2 to 147.848 |
| Index ranges | −6 ≤ h ≤ 6, −22 ≤ k ≤ 23, −14 ≤ l ≤ 14 | −5 ≤ h ≤ 5, −14 ≤ k ≤ 14, −15 ≤ l ≤ 14 |
| Reflections collected | 10,272 | 12,224 |
| Independent reflections | 2231 [Rint = 0.0592, Rsigma = 0.0406] | 2621 [Rint = 0.0800, Rsigma = 0.0449] |
| Data/restraints/parameters | 2231/0/160 | 2621/141/224 |
| Goodness-of-fit on F2 | 1.056 | 1.063 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0579, wR2 = 0.1506 | R1 = 0.0527, wR2 = 0.1331 |
| Final R indexes [all data] | R1 = 0.0898, wR2 = 0.1815 | R1 = 0.0806, wR2 = 0.1628 |
| Largest diff. peak/hole/eÅ−3 | 0.14/−0.24 | 0.29/−0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germonpré, S.; Bhandary, S.; Van Hecke, K. Two Cocrystals of Phenazine with Different Phenylboronic Acids. Molbank 2025, 2025, M2036. https://doi.org/10.3390/M2036
Germonpré S, Bhandary S, Van Hecke K. Two Cocrystals of Phenazine with Different Phenylboronic Acids. Molbank. 2025; 2025(3):M2036. https://doi.org/10.3390/M2036
Chicago/Turabian StyleGermonpré, Stijn, Subhrajyoti Bhandary, and Kristof Van Hecke. 2025. "Two Cocrystals of Phenazine with Different Phenylboronic Acids" Molbank 2025, no. 3: M2036. https://doi.org/10.3390/M2036
APA StyleGermonpré, S., Bhandary, S., & Van Hecke, K. (2025). Two Cocrystals of Phenazine with Different Phenylboronic Acids. Molbank, 2025(3), M2036. https://doi.org/10.3390/M2036







