A Pyrene-Anchored Nickel N-Heterocyclic Carbene–Isoquinoline Complex Promotes CO2 Reduction
Abstract
1. Introduction
2. Results
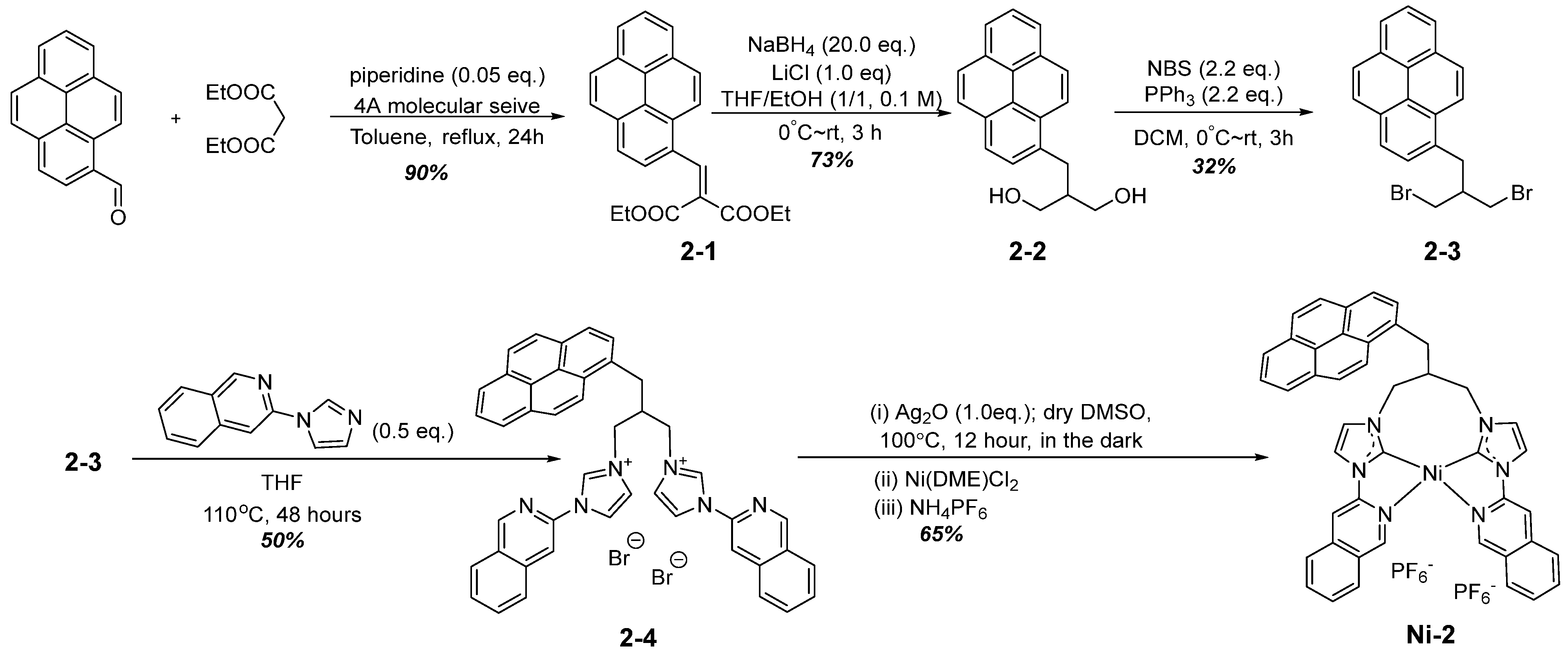
2.1. Synthesis
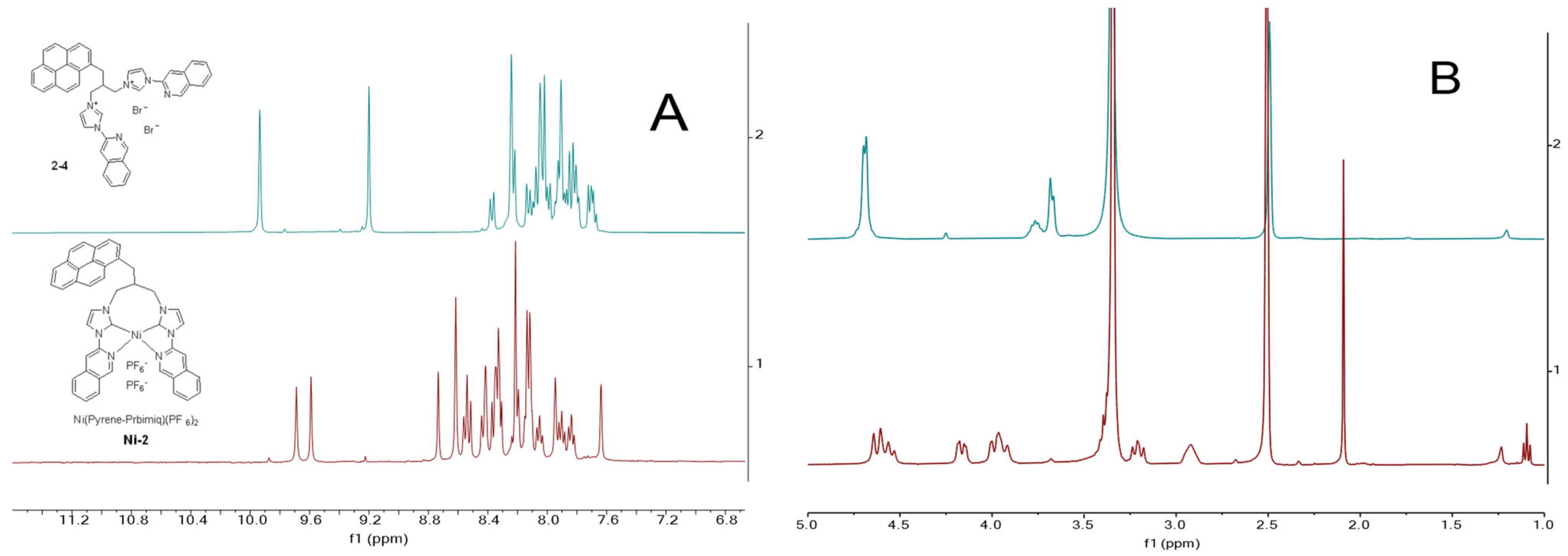
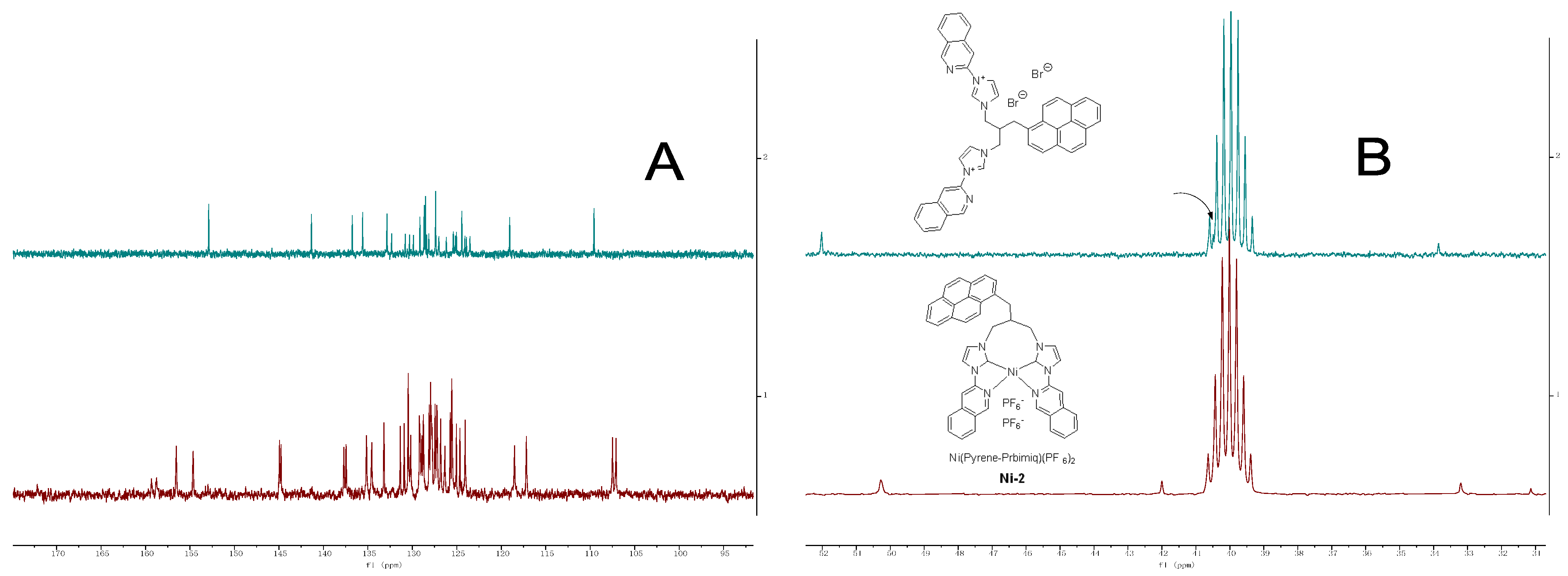
2.2. NMR Characterization
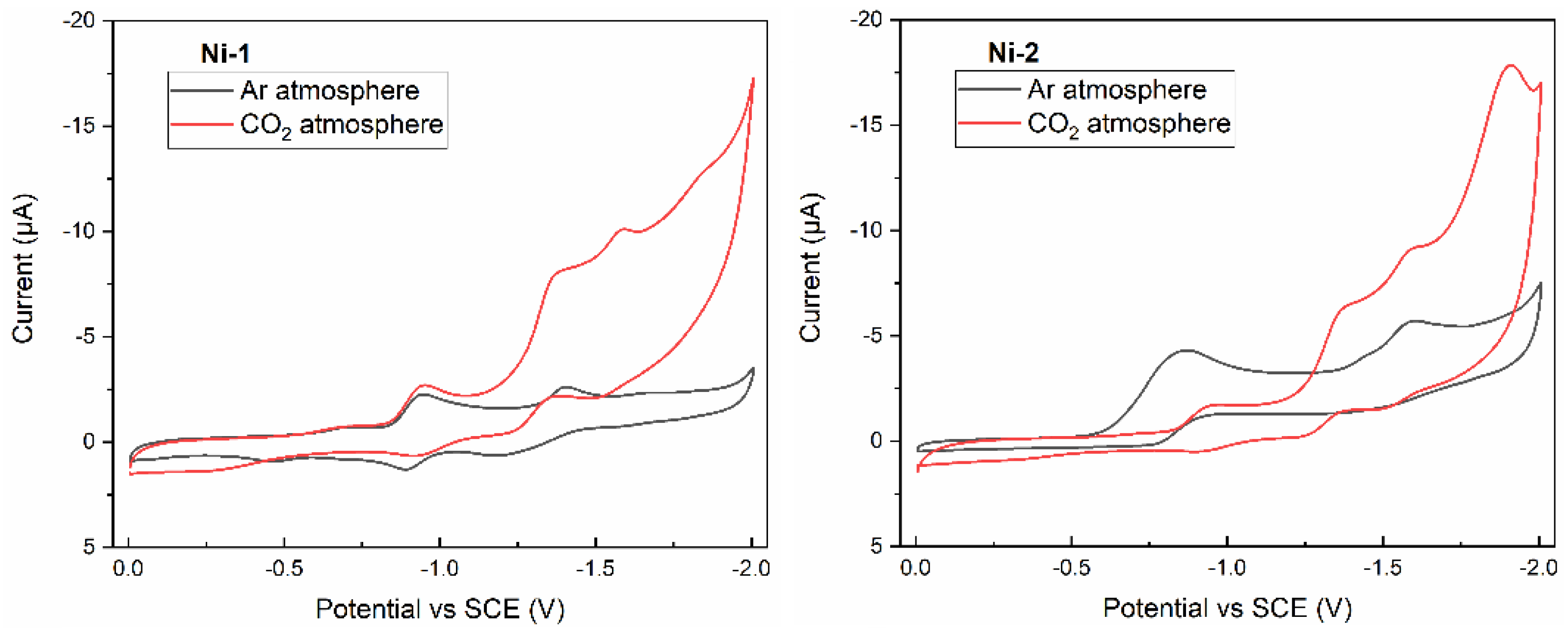
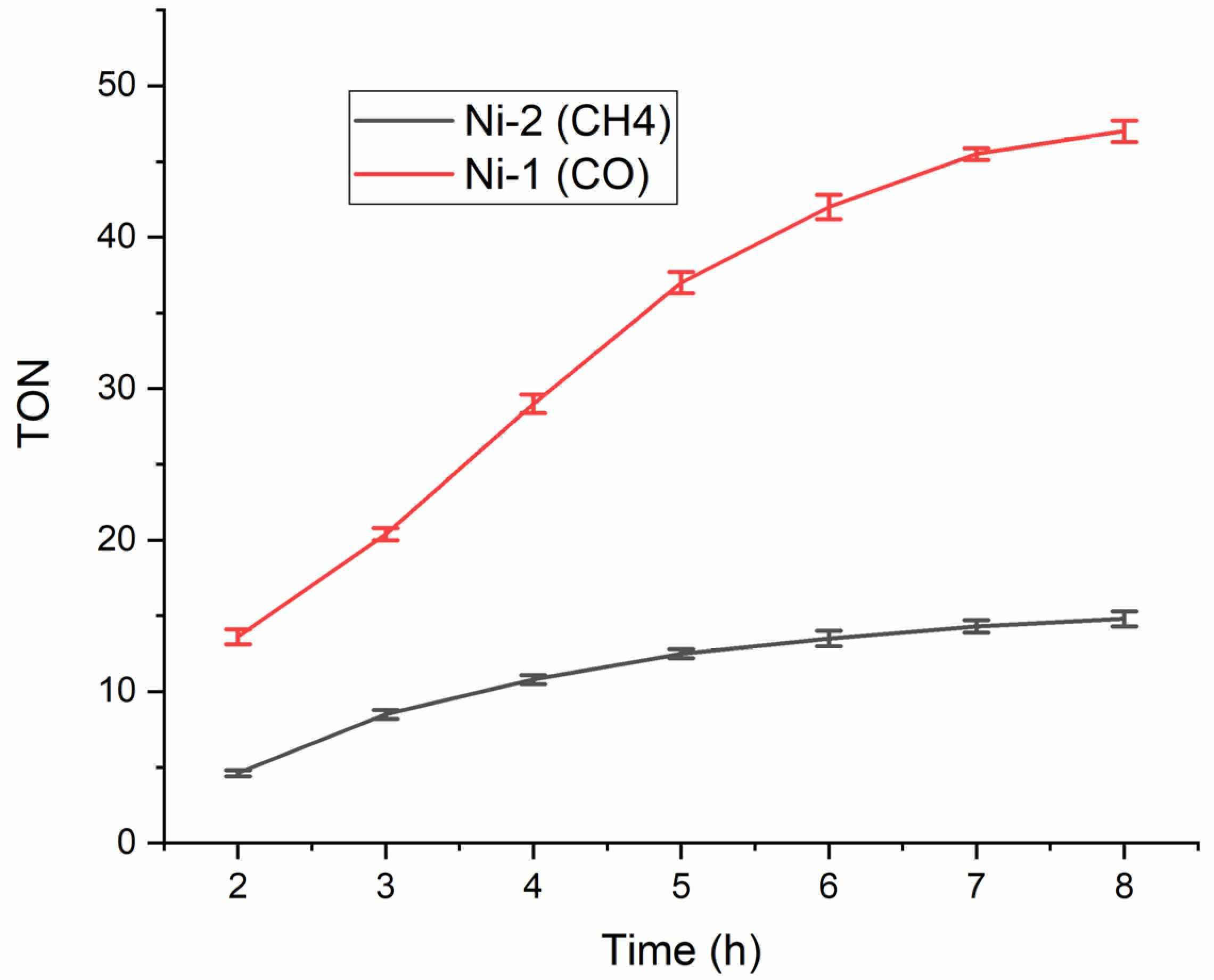
2.3. Evaluation of Ni−2 for Electrocatalytic CO2 Reduction
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, Q.; Li, C.-Y.; Hou, Z.-W.; Wang, L. Photoredox-Catalyzed Defluorinative Carboxylation of gem-Difluorostyrenes with Formate Salt. Org. Let. 2024, 26, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Meyer, T.J.; Brookhart, M. Selective electrocatalytic reduction of carbon dioxide to formate by a water-soluble iridium pincer catalyst. Chem. Sci. 2013, 4, 3497–3502. [Google Scholar] [CrossRef]
- Feng, D.; Geng, X.; Zuo, L.; Li, Z.; Wang, L. Radical-Polar Crossover Bicyclization Enables a Modular Synthesis of Saturated Bicyclic Amines. Adv. Sci. 2025, 12, 2501310. [Google Scholar] [CrossRef]
- Angamuthu, R.; Byers, P.; Lutz, M.; Spek, A.L.; Bouwman, E. Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex. Science 2010, 327, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef]
- Shi, L.-L.; Li, M.; You, B.; Liao, R.-Z. Theoretical Study on the Electro-Reduction of Carbon Dioxide to Methanol Catalyzed by Cobalt Phthalocyanine. Inorg. Chem. 2022, 61, 16549–16564. [Google Scholar] [CrossRef]
- Thoi, V.S.; Chang, C.J. Nickel N-heterocyclic carbene–pyridine complexes that exhibit selectivity for electrocatalytic reduction of carbon dioxide over water. Chem. Commun. 2011, 47, 6578–6580. [Google Scholar] [CrossRef]
- Thoi, V.S.; Kornienko, N.; Margarit, C.G.; Yang, P.; Chang, C.J. Visible-Light Photoredox Catalysis: Selective Reduction of Carbon Dioxide to Carbon Monoxide by a Nickel N-Heterocyclic Carbene–Isoquinoline Complex. J. Am. Chem. Soc. 2013, 135, 14413–14424. [Google Scholar] [CrossRef]
- Reuillard, B.; Ly, K.H.; Rosser, T.E.; Kuehnel, M.F.; Zebger, I.; Reisner, E. Tuning Product Selectivity for Aqueous CO2 Reduction with a Mn(bipyridine)-pyrene Catalyst Immobilized on a Carbon Nanotube Electrode. J. Am. Chem. Soc. 2017, 139, 14425–14435. [Google Scholar] [CrossRef]
- Sinha, S.; Sonea, A.; Shen, W.; Hanson, S.S.; Warren, J.J. Heterogeneous Aqueous CO2 Reduction Using a Pyrene-Modified Rhenium(I) Diimine Complex. Inorg. Chem. 2019, 58, 10454–10461. [Google Scholar] [CrossRef] [PubMed]
- Gonell, S.; Peris, E. Pyrene-Based Mono- and Di-N-Heterocyclic Carbene Ligand Complexes of Ruthenium for the Preparation of Mixed Arylated/Alkylated Arylpyridines. ACS Catal. 2014, 4, 2811–2817. [Google Scholar] [CrossRef]
- Nuevo, D.; Poyatos, M.; Peris, E. A Dinuclear Au(I) Complex with a Pyrene-di-N-heterocyclic Carbene Linker: Supramolecular and Catalytic Studies. Organometallics 2018, 37, 3407–3411. [Google Scholar] [CrossRef]
- Huang, S.; Hong, X.; Sun, Y.; Cui, H.-Z.; Zhou, Q.; Lin, Y.-J.; Hou, X.-F. Ru(II)-PBTNNXN complex bearing functional 2-(pyridin-2-yl)benzo[d]thiazole ligand catalyzed α-alkylation of nitriles with alcohols. Appl. Organomet. Chem. 2020, 34, e5451. [Google Scholar] [CrossRef]
- Chen, X.; Fang, S.-J.; Zhou, Q.; Huang, W.; Liu, Q.-L.; Wang, L. Cu(ii)-catalyzed synthesis of N-sulfonated quinolin-2(1H)-one-3-carboxamides. Org. Biomol. Chem. 2024, 22, 9209–9213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, H.-Y.; Zhou, Y.; Wei, J.-R.; Wang, L. Cu(ii)-Mediated aerobic oxidative synthesis of sulfonated chromeno[4,3-c]pyrazol-4(2H)-ones. Org. Biomol. Chem. 2022, 20, 5575–5581. [Google Scholar] [CrossRef]
- Gyton, M.R.; Leforestier, B.; Chaplin, A.B. Rhodium(III) and Iridium(III) Complexes of a NHC-Based Macrocycle: Persistent Weak Agostic Interactions and Reactions with Dihydrogen. Organometallics 2018, 37, 3963–3971. [Google Scholar] [CrossRef]
- Vidal, I.; Melchor, S.; Alkorta, I.; Elguero, J.; Sundberg, M.R.; Dobado, J.A. On the Existence of α-Agostic Bonds: Bonding Analyses of Titanium Alkyl Complexes. Organometallics 2006, 25, 5638–5647. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, Q.; Li, C.; Wang, L. Syntheses and Structural Characterizations of Benzoxazolyl N-Heterocyclic Carbene-Palladium Complexes and Their Applications. Chin. J. Org. Chem. 2024, 44, 1957–1966. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, J.; Luo, N.; Huang, J. Recent advances in the methylsulfonylation. Acta Chim. Sin. 2025, 83, 274–286. [Google Scholar] [CrossRef]
- Mu, X.; Niu, Y.; Guan, M.; Chen, H.; Wang, L. Pd-Catalyzed Regio- and Stereoselective Synthesis of Tertiary Enamides via a Three-Component Coupling of 2-Arylaziridines with Diazo Esters and Cyclic Ethers. Org. Let. 2025, 27, 5135–5140. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Zhang, X.; Chen, W.; Fu, S.; Wang, D. Synthesis and Structural Characterization of Nickel(II) Complexes Supported by Pyridine-Functionalized N-Heterocyclic Carbene Ligands and Their Catalytic Acitivities for Suzuki Coupling. Organometallics 2007, 26, 6636–6642. [Google Scholar] [CrossRef]
- Kosugi, K.; Akatsuka, C.; Iwami, H.; Kondo, M.; Masaoka, S. Iron-Complex-Based Supramolecular Framework Catalyst for Visible-Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2023, 145, 10451–10457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xue, D.; Qi, H.; Zhang, C. Twisted configuration pyrene derivative: Exhibiting pure blue monomer photoluminescence and electrogenerated chemiluminescence emissions in non-aqueous media. RSC Adv. 2017, 7, 22882–22891. [Google Scholar] [CrossRef]
- Sastre, F.; Puga, A.V.; Liu, L.; Corma, A.; García, H. Complete Photocatalytic Reduction of CO2 to Methane by H2 under Solar Light Irradiation. J. Am. Chem. Soc. 2014, 136, 6798–6801. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-h.; Moon, D.J. CO2 Reforming of Methane over Ni0/La2O3 Catalyst Without Reduction Step: Effect of Calcination Atmosphere. Top. Catal. 2017, 60, 697–705. [Google Scholar] [CrossRef]
- Jian, H.; Lu, M.; Zheng, H.; Yan, S.; Wang, M. Electrochemical Water Oxidation and CO2 Reduction with a Nickel Molecular Catalyst. Molecules 2024, 29, 578. [Google Scholar] [CrossRef]
- Bair, K.W.; Tuttle, R.L.; Knick, V.C.; Cory, M.; McKee, D.D. [(1-Pyrenylmethyl)amino] alcohols, a new class of antitumor DNA intercalators. Discovery and initial amine side chain structure-activity studies. J. Med. Chem. 1990, 33, 2385–2393. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, H.; Geng, M.; Kuang, J.; Ma, Y. Sodium Iodide-Promoted Construction of Fully Substituted 4-Sulfenyl-5-aminopyrazole Derivatives. J. Org. Chem. 2025, 90, 7396–7405. [Google Scholar] [CrossRef]
- Barashkov, N.N. The Synthesis and Luminescence-spectroscopic Properties of Fluorescent Condensation Polymers. Russ. Chem. Rev. 1985, 54, 690–709. [Google Scholar] [CrossRef]
- Masotti, H.; Wallet, J.C.; Peiffer, G.; Petit, F.; Mortreux, A.; Buono, G. Dimerisation de monoolefines catalysee par des complexes du nickel et du cobalt generes electrochimiquement. J. Organomet. Chem. 1986, 308, 241–252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yu, L.-L.; Chen, S.-Y.; Wang, T.; Zhou, Q. A Pyrene-Anchored Nickel N-Heterocyclic Carbene–Isoquinoline Complex Promotes CO2 Reduction. Molbank 2025, 2025, M2035. https://doi.org/10.3390/M2035
Chen X, Yu L-L, Chen S-Y, Wang T, Zhou Q. A Pyrene-Anchored Nickel N-Heterocyclic Carbene–Isoquinoline Complex Promotes CO2 Reduction. Molbank. 2025; 2025(3):M2035. https://doi.org/10.3390/M2035
Chicago/Turabian StyleChen, Xue, Li-Li Yu, Shu-Ying Chen, Tong Wang, and Quan Zhou. 2025. "A Pyrene-Anchored Nickel N-Heterocyclic Carbene–Isoquinoline Complex Promotes CO2 Reduction" Molbank 2025, no. 3: M2035. https://doi.org/10.3390/M2035
APA StyleChen, X., Yu, L.-L., Chen, S.-Y., Wang, T., & Zhou, Q. (2025). A Pyrene-Anchored Nickel N-Heterocyclic Carbene–Isoquinoline Complex Promotes CO2 Reduction. Molbank, 2025(3), M2035. https://doi.org/10.3390/M2035








