A Neutral Heteroleptic Cu(I) Complex with Diimine and Diphosphine Ligands
Abstract
1. Introduction
2. Results and Discussion
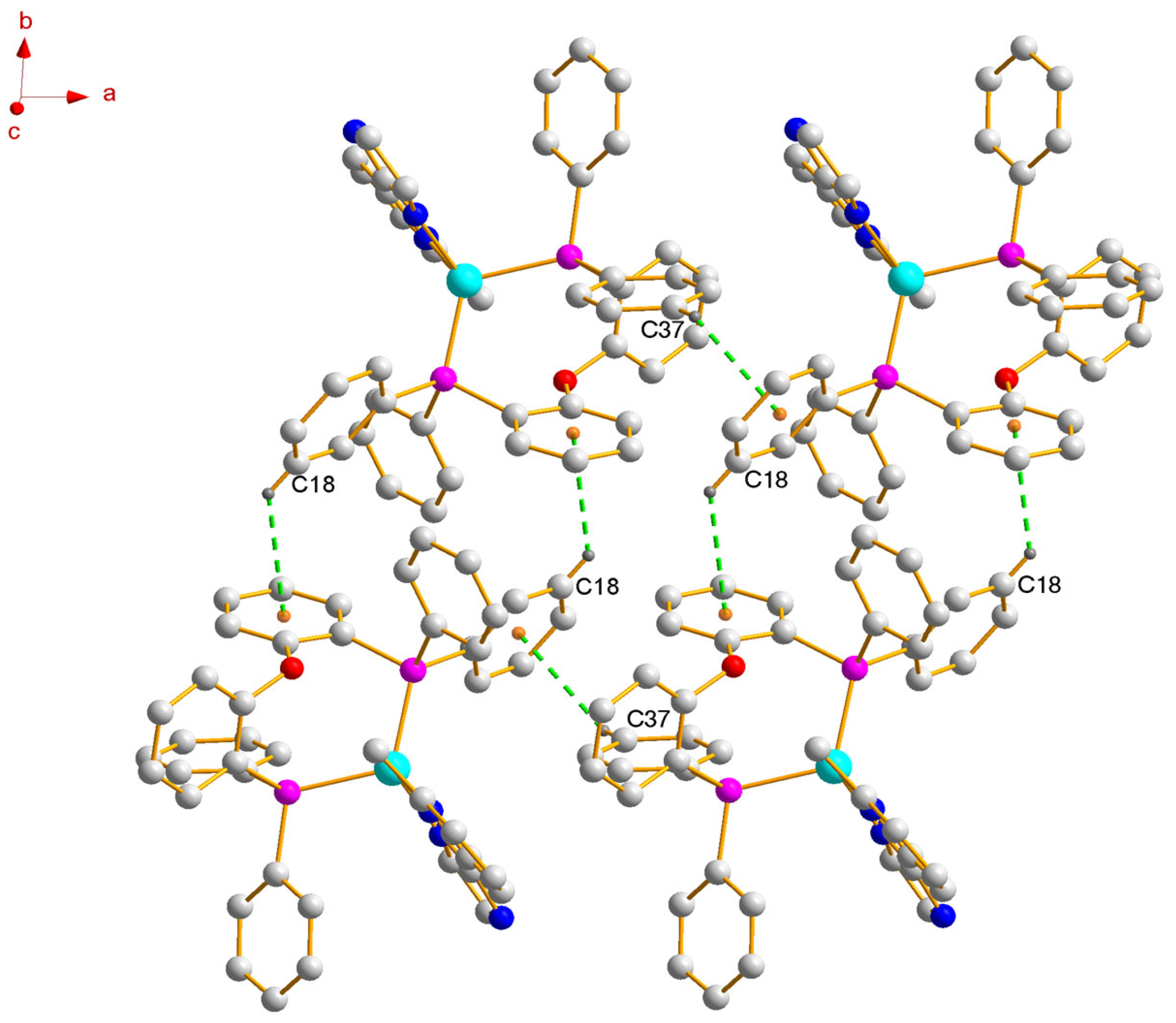
2.1. Synthesis and Single-Crystal Structure
2.2. NMR and MALDI-TOF-MS Spectra Analysis
3. Materials and Methods
3.1. Synthesis Procedure for [(Immp)Cu(POP)]
3.2. X-Ray Crystal Structure Analysis of [(Immp)Cu(POP)]
3.3. FT-IR, NMR, and MALDI-TOF-MS Analysis of [(Immp)Cu(POP)]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Xu, C.; Ji, B.; Zheng, C.; Zhang, X. Excellent deep-blue emitting materials based on anthracene derivatives for non-doped organic light-emitting diodes. Opt. Mater. 2016, 58, 260–267. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Liu, C.-L.; Zheng, C.-J.; Wang, W.-Z.; Xu, C.; Zhu, M.; Ji, B.-M.; Li, F.; Zhang, X.-H. Efficient violet non-doped organic light-emitting device based on a pyrene derivative with novel molecular structure. Org. Electron. 2015, 23, 179–185. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal-organic complexes for optoelectronic applications. Chem. Soc. Rew. 2014, 43, 3259–3302. [Google Scholar] [CrossRef]
- Farokhi, A.; Lipinski, S.; Cavinato, L.M.; Shahroosvand, H.; Pashaei, B.; Karimi, S.; Bellani, S.; Bonaccorso, F.; Costa, R.D. Metal complex-based TADF: Design, characterization, and lighting devices. Chem. Soc. Rew. 2025, 54, 266–340. [Google Scholar] [CrossRef]
- Hofbeck, T.; Monkowius, U.; Yersin, H. Highly efficient luminescence of Cu(I) compounds: Thermally activated delayed fluorescence combined with short-lived phosphorescence. J. Am. Chem. Soc. 2015, 137, 399–404. [Google Scholar] [CrossRef]
- Liang, D.; Chen, X.-L.; Liao, J.-Z.; Hu, J.-Y.; Jia, J.-H.; Lu, C.-Z. Highly efficient cuprous complexes with thermally activated delayed fluorescence for solution-processed organic light-emitting devices. Inorg. Chem. 2016, 55, 7467–7475. [Google Scholar] [CrossRef]
- Liu, L.-P.; Li, Q.; Xiang, S.-P.; Liu, L.; Zhong, X.-X.; Liang, C.; Li, G.H.; Hayat, T.; Alharbi, N.S.; Li, F.-B.; et al. Near saturated red emitters: Four-coordinate copper(I) halide complexes containing 8-(diphenylphosphino)quinoline and 1-(diphenylphosphino)naphthalene ligands. Dalton Trans. 2018, 47, 9294–9302. [Google Scholar] [CrossRef]
- Lin, S.; Peng, Q.; Ou, Q.; Shuai, Z. Strong solid-state fluorescence induced by restriction of the coordinate bond bending in two-coordinate copper(I)-carbene complexes. Inorg. Chem. 2019, 58, 14403–14409. [Google Scholar] [CrossRef]
- Klein, M.; Rau, N.; Wende, M.; Sundermeyer, J.; Cheng, G.; Che, C.-M.; Schinabeck, A.; Yersin, H. Cu(I) and Ag(I) complexes with a new type of rigid tridentate N,P,P-ligand for thermally activated delayed fluorescence and OLEDs with high external quantum efficiency. Chem. Mater. 2020, 32, 10365–10382. [Google Scholar] [CrossRef]
- Xu, K.; Chen, B.-L.; Yang, F.; Liu, L.; Zhong, X.-X.; Wang, L.; Zhu, X.-J.; Li, F.-B.; Wong, W.-Y.; Qin, H.-M. Largely color-tuning prompt and delayed fluorescence: Dinuclear Cu(I) halide complexes with tert-amines and phosphines. Inorg. Chem. 2021, 60, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Pauker, C.; Santander-Nelli, M.; Dreyse, P. Thermally activated delayed fluorescence in luminescent cationic copper(i) complexes. RSC Adv. 2022, 12, 10653–10674. [Google Scholar] [CrossRef] [PubMed]
- He, H.-F.; Zhang, J.-K.; Wu, X.-Y.; Zhao, F.; Huang, Y.-Z.; Wang, M.-C.; Feng, C.-X.; Mao, D.-R.; Huang, X.-L.; Hu, Y.-F. Hypso- or bathochromic phosphorescent mechanochromic mononuclear Cu(I) complexes with a bis(2-diphenylphosphinophenyl)ether auxiliary ligand. Dalton Trans. 2023, 52, 13358–13366. [Google Scholar] [CrossRef] [PubMed]
- Ruduss, A.; Jece, A.; Stucere, K.A.; Chen, K.-W.; Turovska, B.; Belyakov, S.; Vembris, A.; Chang, C.-H.; Traskovskis, K. Sulfonyl-functionalized benzo[d]imidazo[5,1-b]thiazole-based carbenes as building blocks for two-coordinate Cu(i) complexes exhibiting fast and efficient thermally activated delayed fluorescence. J. Mater. Chem. C 2024, 12, 2968–2980. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhu, R.; Liu, L.; Zhong, X.-X.; Li, F.-B.; Zhou, G.; Qin, H.-M. High-performance TADF-OLEDs utilizing copper(i) halide complexes containing unsymmetrically substituted thiophenyl triphosphine ligands. Inorg. Chem. Front. 2025, 12, 1139–1155. [Google Scholar] [CrossRef]
- Felder, D.; Nierengarten, J.-F.; Barigelletti, F.; Ventura, B.; Armaroli, N. Highly luminescent Cu(I)-phenanthroline complexes in rigid matrix and temperature dependence of the photophysical properties. J. Am. Chem. Soc. 2001, 123, 6291–6299. [Google Scholar] [CrossRef]
- Chai, C.; Xu, S.; Wang, J.; Zhao, F.; Xia, H.; Wang, Y. Synthesis, photophysical properties and DFT studies of the pyridine imidazole (PyIm) Cu(I) complexes: Impact of the pyridine ring functionalized by different substituents. Inorg. Chim. Acta 2019, 488, 34–40. [Google Scholar] [CrossRef]
- Saito, K.; Arai, T.; Takahaki, N.; Tsukuda, T.; Tsubomura, T. A series of luminescent Cu(I) mixed-ligand complexes containing 2,9-dimethyl-1,10-phenanthroline and simple diphosphine ligands. Dalton Trans. 2006, 4444–4448. [Google Scholar] [CrossRef]
- Bergmann, L.; Braun, C.; Nieger, M.; Bräse, S. The coordination- and photochemistry of copper(i) complexes: Variation of N^N ligands from imidazole to tetrazole. Dalton Trans. 2018, 47, 608–621. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, C.; Wang, W.; Xu, C.; Ji, B.; Zhang, X. Synthesis, Structure, and Photophysical Properties of Two Four-Coordinate CuI-NHC Complexes with Efficient Delayed Fluorescence. Inorg. Chem. 2016, 55, 2157–2164. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Fu, W.; Xu, C.; Ji, B. Four-coordinate Cu(I) complexes supported by N-heterocyclic carbene ligands bearing electron-donating/withdrawing groups: Synthesis, structures and photophysical properties. J. Lumin. 2018, 204, 618–625. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Xu, C.; Ji, B. Synthesis, Structures, and Photophysical Properties of Novel Four-Coordinate Cu(I) Complexes Supported by Chelating N-Heterocyclic Carbene Ligands. Front. Chem. 2019, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z. Synthesis, structures and photophysical properties of two new Cu(I) complexes. Z. Naturforsch. B 2023, 78, 537–542. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive mono- and polynuclear Cu(i)-phenanthrolines. A viable alternative to Ru(ii)-polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Scaltrito, D.V.; Thompson, D.W.; O’Callaghan, J.A.; Meyer, G.J. MLCT excited states of cuprous bis-phenanthroline coordination compounds. Coord. Chem. Rev. 2000, 208, 243–1016. [Google Scholar] [CrossRef]
- Santander-Nelli, M.; Cortés-Arriagada, D.; Sanhueza, L.; Dreyse, P. Dependence between luminescence properties of Cu(i) complexes and electronic/structural parameters derived from steric effects. New J. Chem. 2022, 46, 10584–10593. [Google Scholar] [CrossRef]
- Sztula, A.; Antal, P.; Nemec, I.; Kubala, M.; Herchel, R. A novel type of heteroleptic Cu(i) complexes featuring nitrogen-rich tetrazine ligands: Syntheses, crystal structures, spectral properties, cyclic voltammetry, and theoretical calculations. Dalton. Trans. 2025, 54, 5944–5952. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]

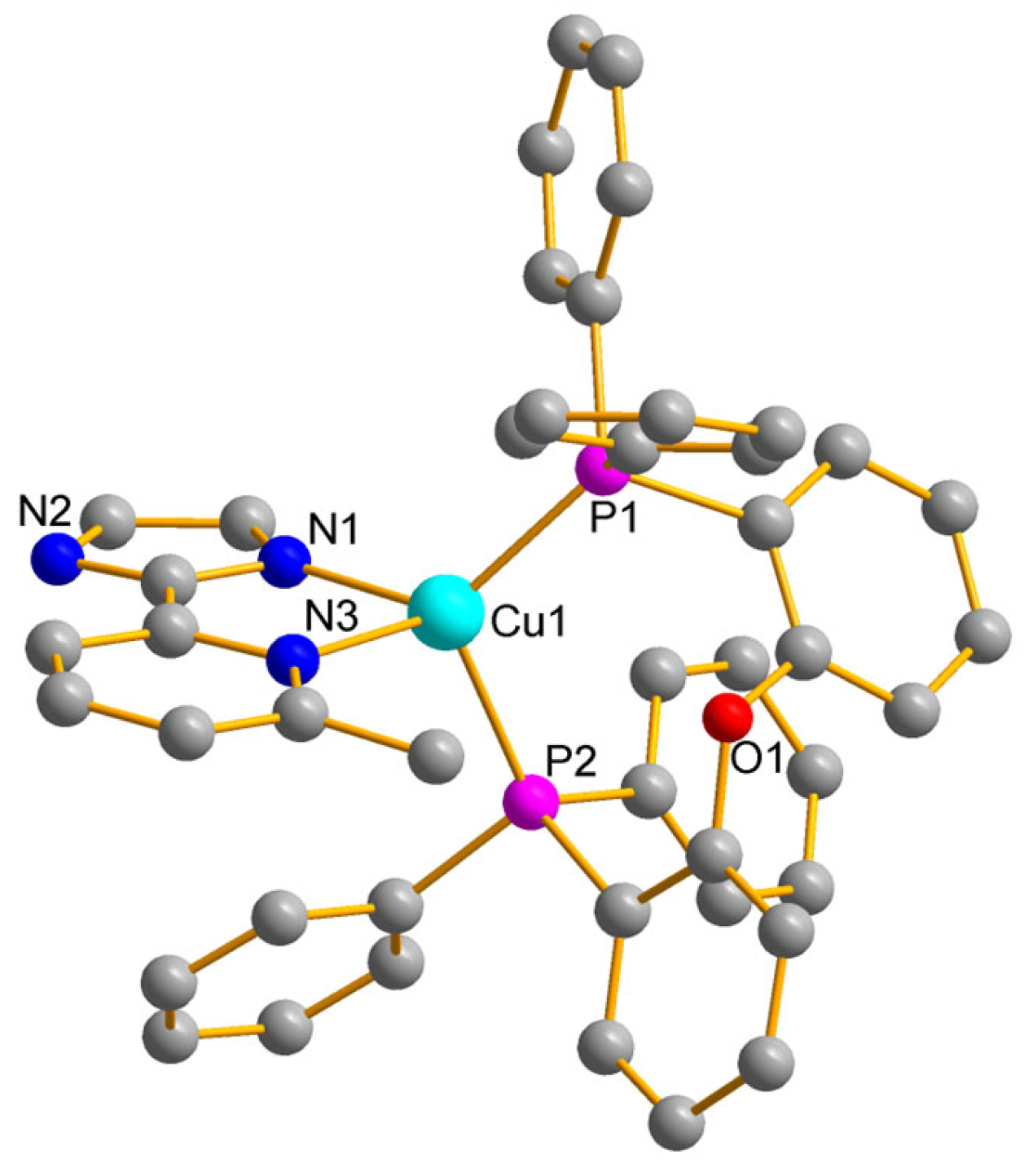
| Bond lengths (Å) | |||
| Cu1-N1 | 2.017(3) | Cu1-N3 | 2.141(3) |
| Cu1-P1 | 2.2477(9) | Cu1-P2 | 2.2948(8) |
| Bond angles (°) | |||
| N1-Cu1-N3 | 81.00(12) | P1-Cu1-P2 | 113.87(3) |
| N1-Cu1-P1 | 123.60(8) | N3-Cu1-P1 | 117.10(7) |
| N1-Cu1-P2 | 107.08(8) | N3-Cu1-P2 | 109.67(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Sheng, R.; Petkovic, M.; Jaśkowska, J.; Wang, Z. A Neutral Heteroleptic Cu(I) Complex with Diimine and Diphosphine Ligands. Molbank 2025, 2025, M2019. https://doi.org/10.3390/M2019
Sun X, Sheng R, Petkovic M, Jaśkowska J, Wang Z. A Neutral Heteroleptic Cu(I) Complex with Diimine and Diphosphine Ligands. Molbank. 2025; 2025(2):M2019. https://doi.org/10.3390/M2019
Chicago/Turabian StyleSun, Xiaojuan, Ruilong Sheng, Marijana Petkovic, Jolanta Jaśkowska, and Zhiqiang Wang. 2025. "A Neutral Heteroleptic Cu(I) Complex with Diimine and Diphosphine Ligands" Molbank 2025, no. 2: M2019. https://doi.org/10.3390/M2019
APA StyleSun, X., Sheng, R., Petkovic, M., Jaśkowska, J., & Wang, Z. (2025). A Neutral Heteroleptic Cu(I) Complex with Diimine and Diphosphine Ligands. Molbank, 2025(2), M2019. https://doi.org/10.3390/M2019







