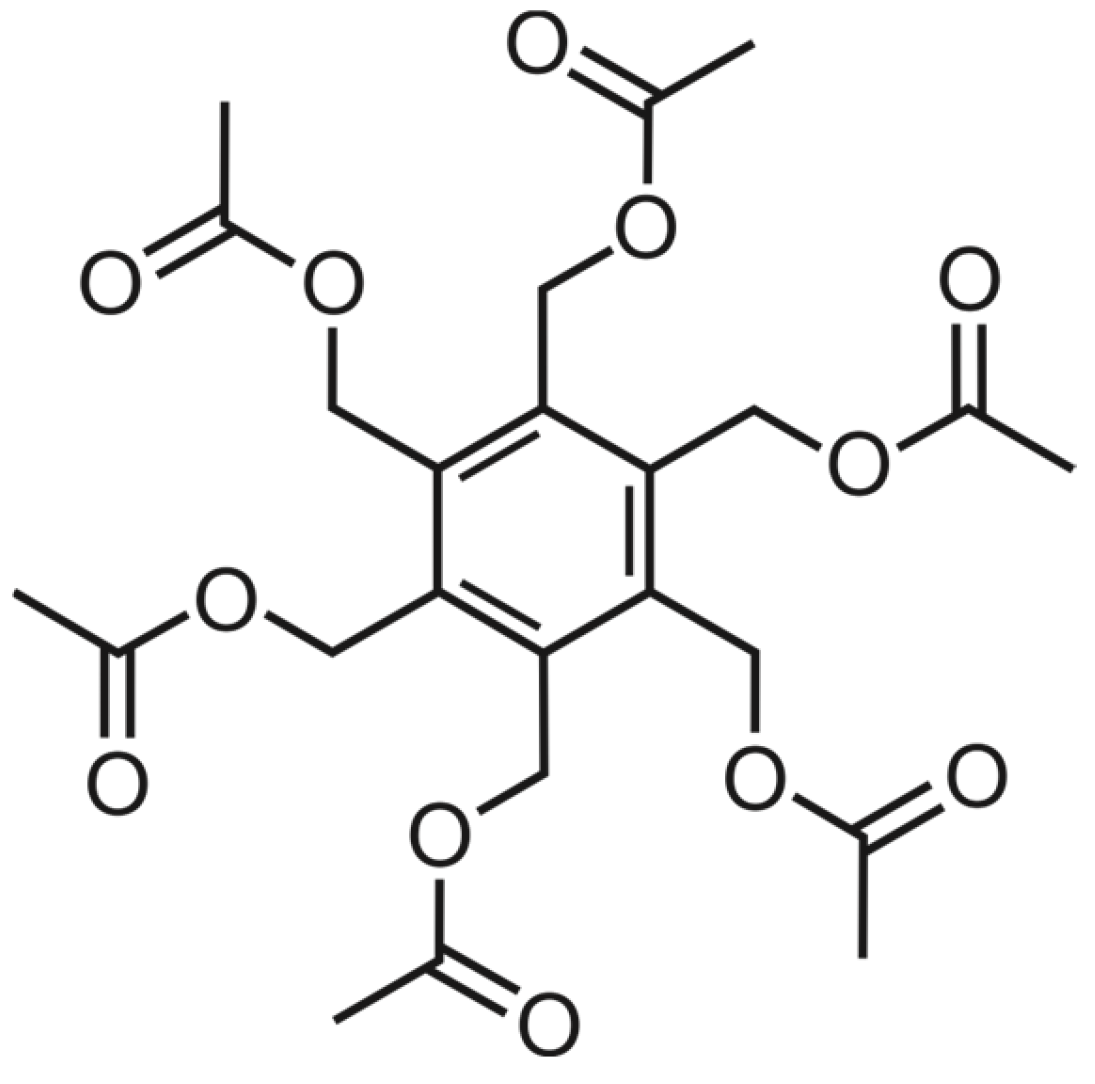
Crystal Structure and Hirshfeld Surface Analysis of Hexakis(acetoxymethyl)benzene
Abstract
1. Introduction
2. Results and Discussion
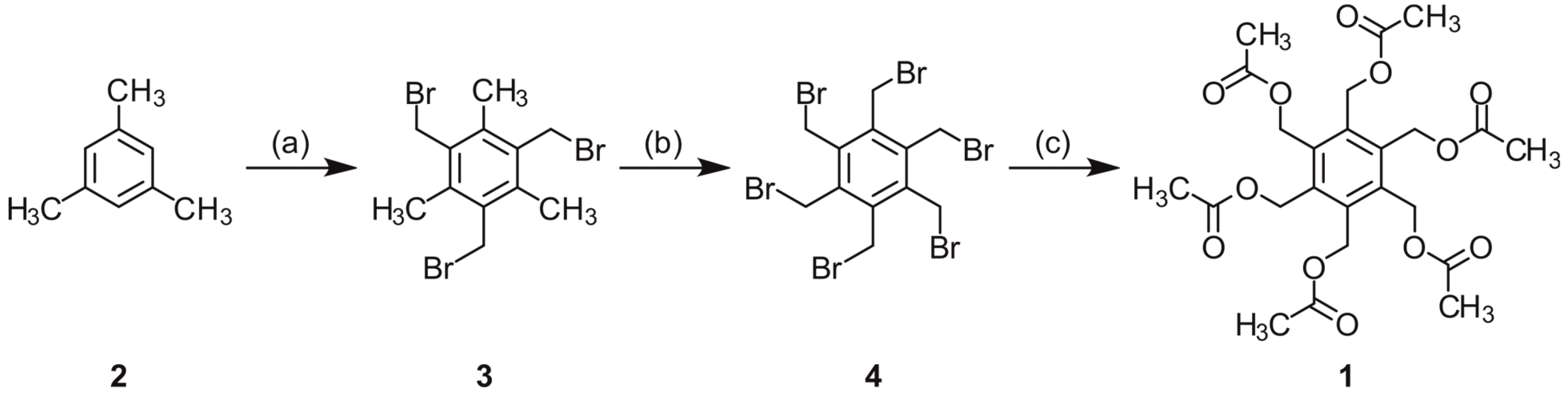
2.1. Synthesis
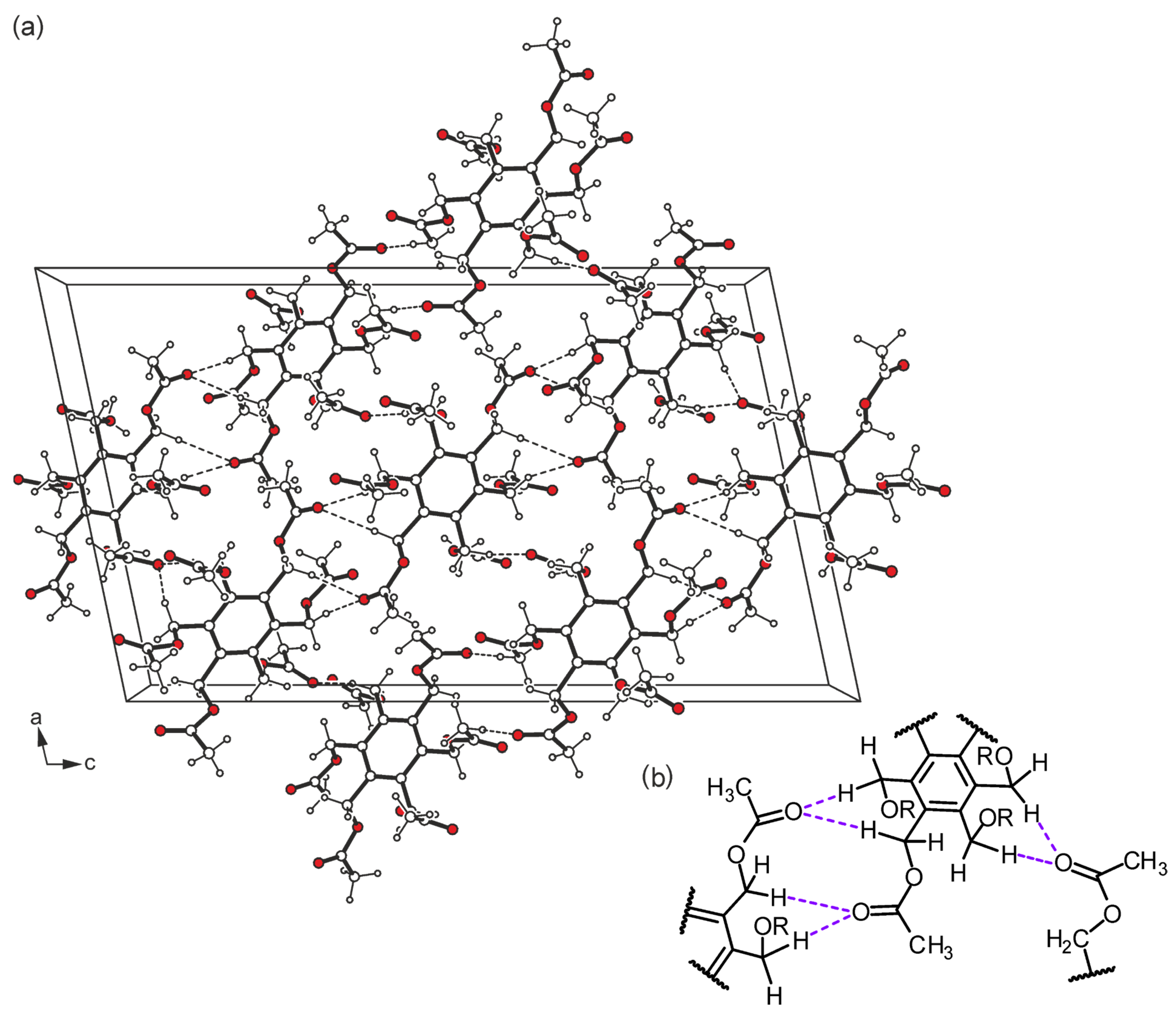
2.2. Structure Elucidation
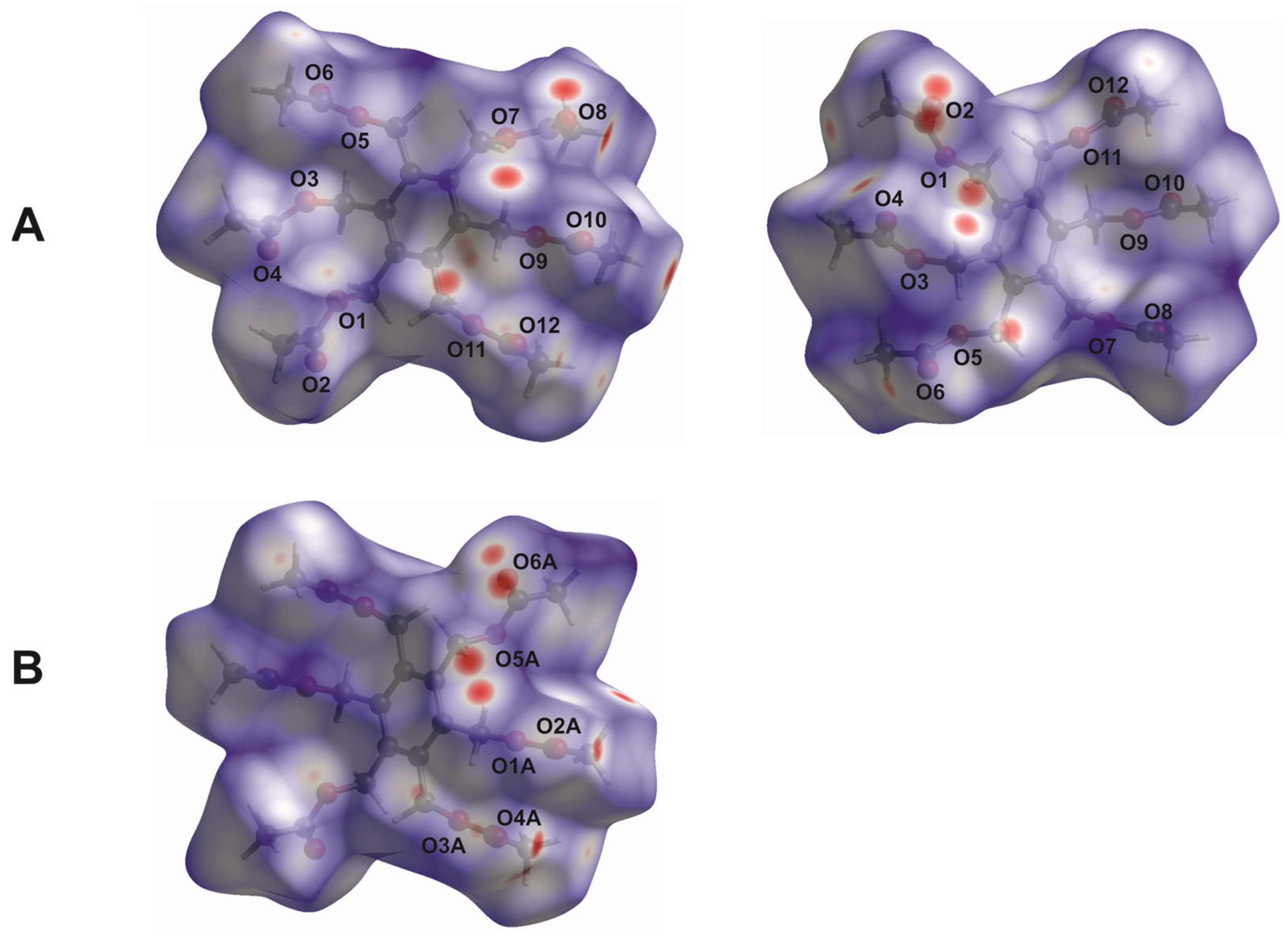
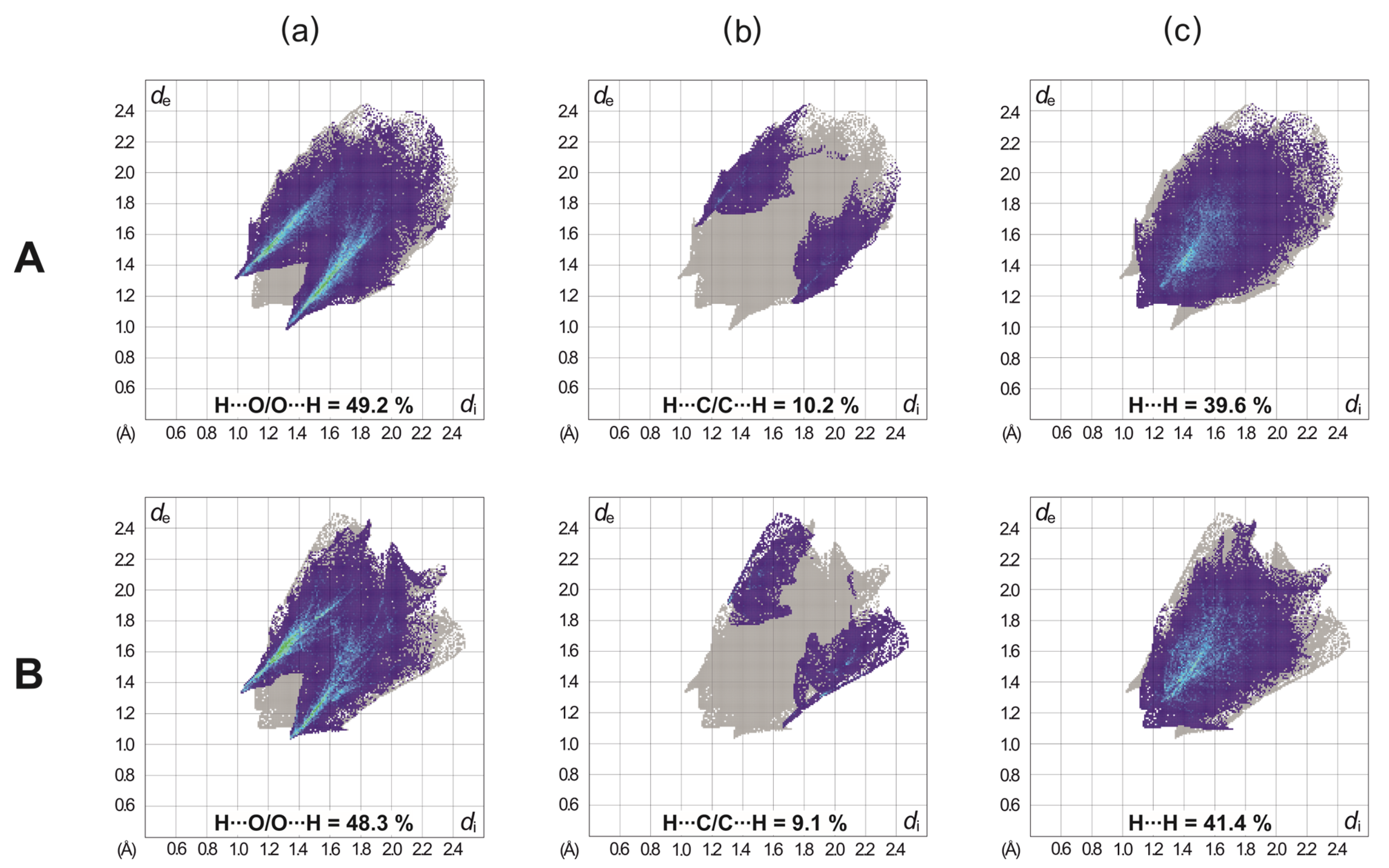
2.3. Hirshfeld Surface Analysis
3. Materials and Methods
3.1. Synthesis
3.2. Crystallization and X-Ray Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- MacNicol, D.D.; Hardy, A.D.U.; Wilson, D.R. Crystal and molecular structure of a ‘hexa-host’ inclusion compound. Nature 1977, 266, 611–612. [Google Scholar] [CrossRef]
- MacNicol, D.D.; Wilson, D.R. New Strategy for the Design of Inclusion Compounds: Discovery of the ‘Hexa-hosts’. J. Chem. Soc. Chem. Commun. 1976, 494–495. [Google Scholar] [CrossRef]
- Vögtle, F.; Weber, E. Krakenmoleküle. Angew. Chem. 1974, 86, 896–898. [Google Scholar] [CrossRef]
- Arunachalam, M.; Ahamed, B.N.; Ghosh, P. Binding of Ammonium Hexafluorophosphate and Cation-Induced Isolation of Unusual Conformers of a Hexapodal Receptor. Org. Lett. 2010, 12, 2742–2745. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Ding, W.; Chen, X.; Wu, L.; Huang, Z.; Zhang, Z. Pyrrole-based acyclic hexapodal aldehyde for anion recognition. Tetrahedron 2024, 165, 134170. [Google Scholar] [CrossRef]
- Koch, N.; Seichter, W.; Mazik, M. Hexapodal pyrazole-based receptors: Complexes with ammonium ions and solvent molecules in the solid state. Tetrahedron 2015, 71, 8965–8974. [Google Scholar] [CrossRef]
- Arunachalam, M.; Ghosh, P. Bistripodand Amide Host for Compartmental Recognition of Multiple Oxyanions. Org. Lett. 2010, 12, 328–331. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dutta, R.; Wong, B.M.; Ghosh, P. Anion directed conformational diversities of an arene based hexa-amide receptor and recognition of the [F4(H2O)6]4− cluster. RSC Adv. 2014, 4, 62689–62693. [Google Scholar] [CrossRef]
- Arunachalam, M.; Ghosh, P. Encapsulation of [F4(H2O)10]4− in a dimeric assembly of an unidirectional arene based hexapodal amide receptor. Chem. Commun. 2011, 22, 6269–6271. [Google Scholar] [CrossRef]
- Chakraborty, S.; Arunachalam, M.; Dutta, R.; Ghosh, P. Arene platform based hexa-amide receptors for anion recognition: Single crystal X-ray structural and thermodynamic studies. RSC Adv. 2015, 5, 48060–48070. [Google Scholar] [CrossRef]
- Förster, S.; Seichter, W.; Weber, E. Synthesis and Structures of Three- and Hexa-armed Benzene Derivatives Featuring Lateral Benzoic Ester and Benzoic Acid Functions. Z. Naturforschung B 2011, 66, 939–946. [Google Scholar] [CrossRef]
- Das, D.; Barbour, L.J. Polymorphism of a Hexa-host: Isolation of Four Different Single-Crystal Phases by Melt Crystallization. J. Am. Chem. Soc. 2008, 130, 14032–14033. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Barbour, L.J. Unusual Conformations of a Hexa-Host Molecule in Solvate Inclusion Compounds. Cryst. Growth Des. 2009, 9, 1599–1604. [Google Scholar] [CrossRef]
- Das, D.; Barbour, L.J. Concomitant formation of two different solvates of a hexa-host from a binary mixture of solvents. Chem. Commun. 2008, 5110–5112. [Google Scholar] [CrossRef]
- Gavette, J.V.; Sargent, A.L.; Allen, W.E. Hydrogen Bonding vs Steric Gearing in a Hexasubstituted Benzene. J. Org. Chem. 2008, 73, 3582–3584. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Steiner, T. Unrolling the hydrogen bond properties of C–H···O interactions. Chem. Commun. 1997, 727–734. [Google Scholar] [CrossRef]
- Steiner, T. Effect of acceptor strength on C–H···O hydrogen bond lengths as revealed by and quantified from crystallographic data. J. Chem. Soc. Chem. Commun. 1994, 2341–2342. [Google Scholar] [CrossRef]
- Steiner, T.; Desiraju, G.R. Distinction between the weak hydrogen bond and the van der Waals interaction. Chem. Commun. 1998, 891–892. [Google Scholar] [CrossRef]
- Desiraju, G.R. C–H···O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem. Commun. 2005, 2995–3001. [Google Scholar] [CrossRef]
- Mazik, M.; Bläser, D.; Boese, R. Intermolecular CH···N/CH···O hydrogen bonds in the crystal structures of α,β-unsaturated ketones carrying a terminal pyridine subunit. Tetrahedron 2001, 57, 5791–5797. [Google Scholar] [CrossRef]
- Mazik, M.; Hartmann, A.; Jones, P.G. Hydrogen and Halogen Bonding in the Crystal Structure of a 1,3,5-Substituted 2,4,6-Triethylbenzene Consisting of Three Phenanthroline Units. Eur. J. Org. Chem. 2010, 2010, 458–463. [Google Scholar] [CrossRef]
- Castellano, R.K. Progress Toward Understanding the Nature and Function of C–H···O Interactions. Curr. Org. Chem. 2004, 8, 845–865. [Google Scholar] [CrossRef]
- Horowitz, S.; Trievel, R.C. Carbon-Oxygen Hydrogen Bonding in Biological Structure and Function. J. Biol. Chem. 2012, 287, 41576–41582. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, Y.; Jones, L.O.; Song, B.; Guo, Q.-H.; Zhang, L.; Qiu, Y.; Feng, Y.; Chen, X.-Y.; Schatz, G.C.; et al. PCage: Fluorescent Molecular Temples for Binding Sugars in Water. J. Am. Chem. Soc. 2021, 143, 15688–15700. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Nakashima, Y.; Tsukamoto, S.; Kurohara, T.; Suzuki, M.; Sakae, Y.; Oda, M.; Okamoto, Y.; Suzuki, T. N+–C–H···O Hydrogen bonds in protein-ligand complexes. Sci. Rep. 2019, 9, 767. [Google Scholar] [CrossRef]
- Scheiner, S. Weak H-bonds. Comparisons of CH···O to NH···O in proteins and PH···N to direct P···N interactions. Phys. Chem. Chem. Phys. 2011, 13, 13860–13872. [Google Scholar] [CrossRef]
- Backer, H.J. L’hexa-hydroxyméthyl-benzène et ses dérivés (composés planradiaires I). Recl. Trav. Chim. Pays-Bas 1935, 54, 833–837. [Google Scholar] [CrossRef]
- Závada, J.; Pánková, M.; Holý, P.; Tichý, M. A Facile Synthesis of Hexakis(bromomethyl)benzene from Mesitylene. Synthesis 1994, 1994, 1132. [Google Scholar] [CrossRef]
- Biali, S.E.; Mislow, K. Barrier to Internal Rotation in l,2-Bis(bromochloromethyl)-3,4,5,6-tetraisopropylbenzene. J. Org. Chem. 1988, 53, 1318–1320. [Google Scholar] [CrossRef]
- Biali, S.E.; Gutiérrez, A.; Mislow, K. Achiral Hexaisopropylbenzene Isotopomers: Analogues of the Achiral Trihydroxyglutaric Acid Diastereomers. J. Org. Chem. 1988, 53, 1316–1318. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Venkatesan, P.; Thamotharan, S.; Ilangovan, A.; Liang, H.; Sundius, T. Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino]prop-2-enoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 625–636. [Google Scholar] [CrossRef]
- APEX2 and SAINT. Bruker AXS Inc.: Madison, WI, USA, 2005.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stapf, M.; Seichter, W.; Mazik, M. Crystal Structure and Hirshfeld Surface Analysis of Hexakis(acetoxymethyl)benzene. Molbank 2025, 2025, M2008. https://doi.org/10.3390/M2008
Stapf M, Seichter W, Mazik M. Crystal Structure and Hirshfeld Surface Analysis of Hexakis(acetoxymethyl)benzene. Molbank. 2025; 2025(2):M2008. https://doi.org/10.3390/M2008
Chicago/Turabian StyleStapf, Manuel, Wilhelm Seichter, and Monika Mazik. 2025. "Crystal Structure and Hirshfeld Surface Analysis of Hexakis(acetoxymethyl)benzene" Molbank 2025, no. 2: M2008. https://doi.org/10.3390/M2008
APA StyleStapf, M., Seichter, W., & Mazik, M. (2025). Crystal Structure and Hirshfeld Surface Analysis of Hexakis(acetoxymethyl)benzene. Molbank, 2025(2), M2008. https://doi.org/10.3390/M2008







