(Z)-4-(Azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one †
Abstract
1. Introduction
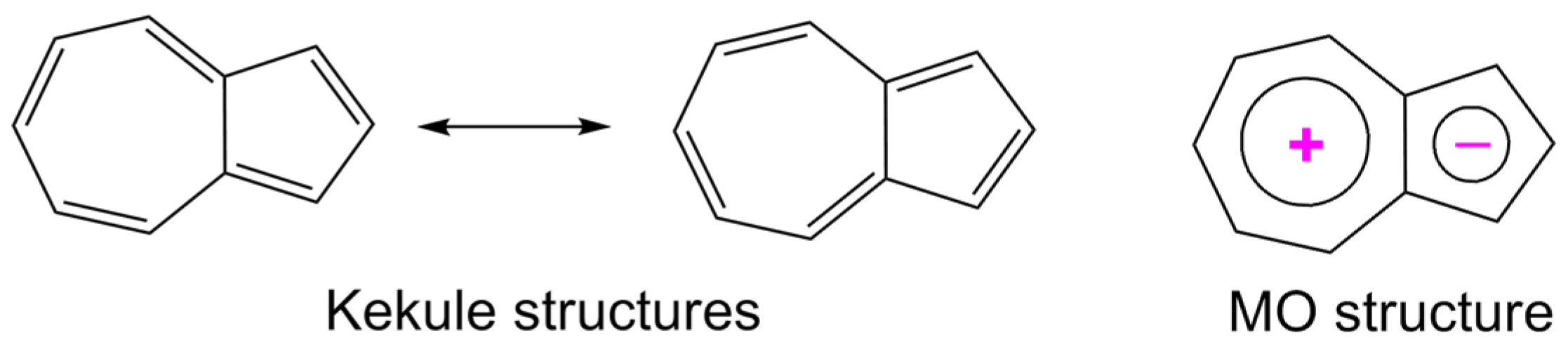
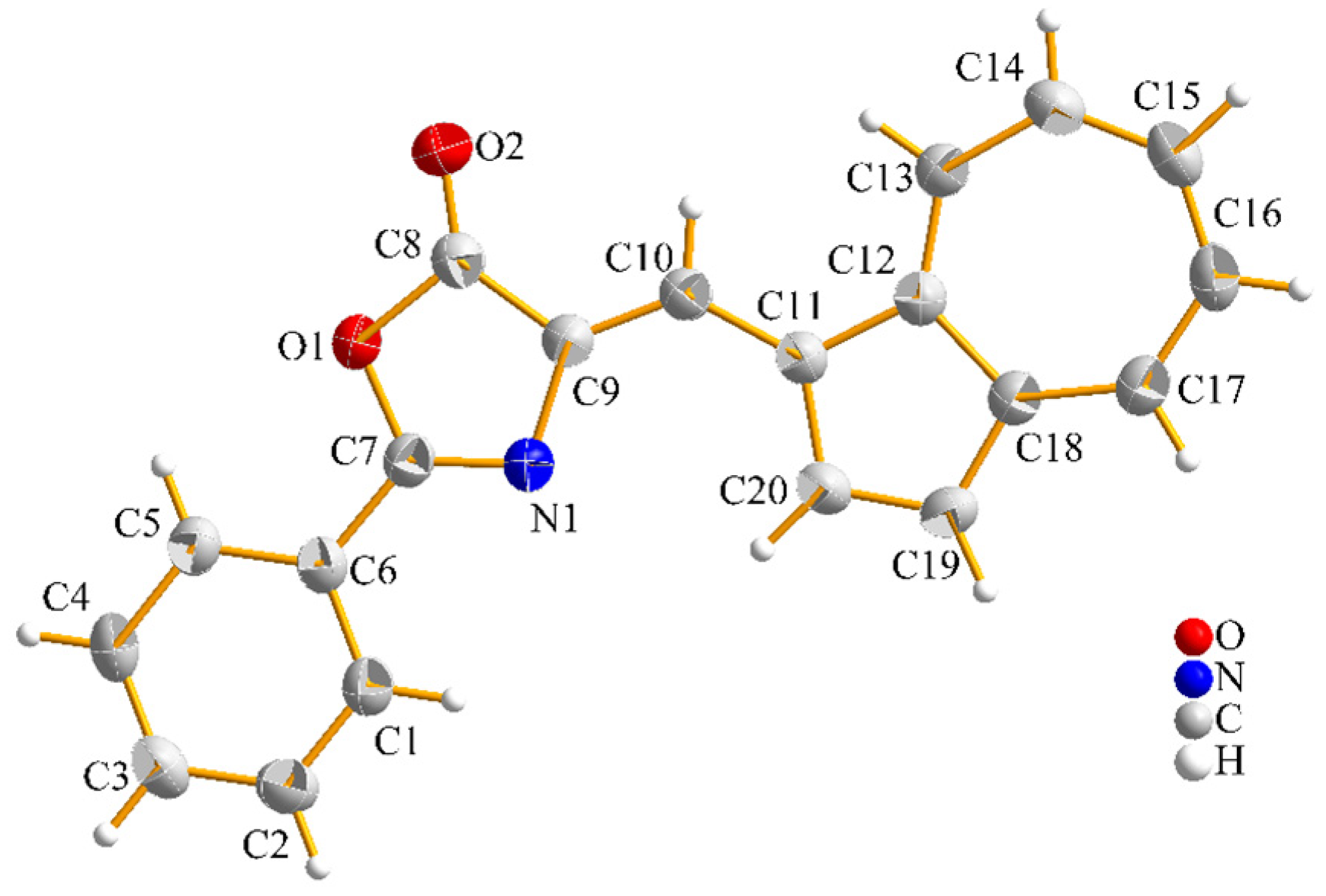
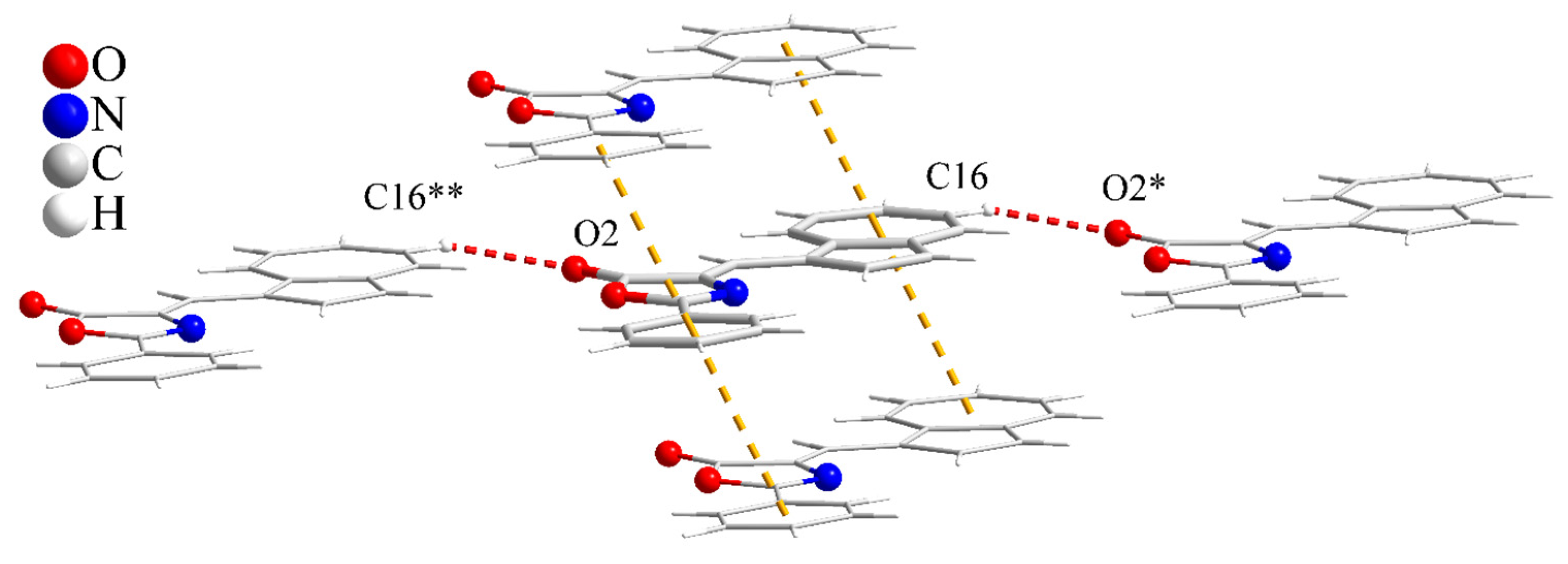
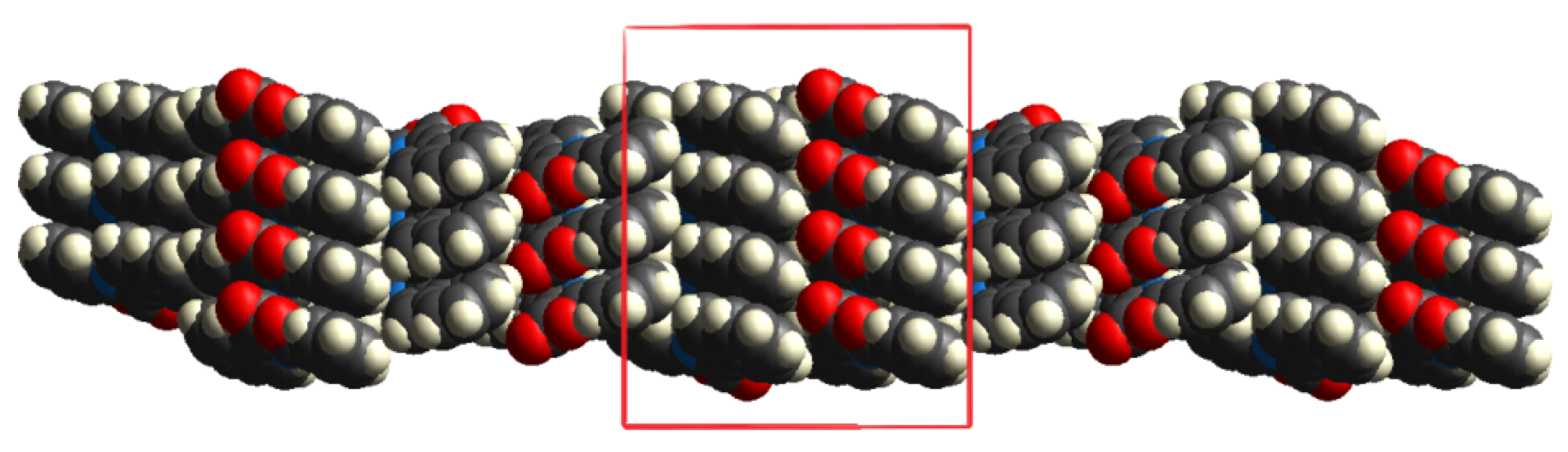
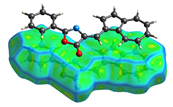
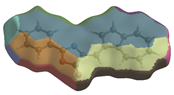
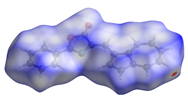
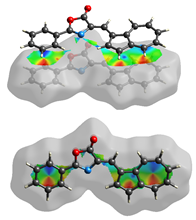
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeller, K.-P. Methoden der Organischen Chemie; G. Thieme Verlag: Stuttgart, NY, USA, 1985; Volume V/2c, pp. 127–416. [Google Scholar]
- Razus, A.C.; Birzan, L. Synthesis of azulenic compounds with a homo- or hetero-atomic double bond at position. Arkivoc 2018, IV, 1–56. [Google Scholar] [CrossRef]
- Zadeh Ghazvini, E.H.; Tang, S.; Woodward, A.; Liu, W.T.; Bondar, M.V.; Belfield, K.D. Chromophoric materials derived from a natural azulene: Syntheses, halochromism and one-photon and two-photon microlithography. J. Mater. Chem. C 2015, 3, 8495–8503. [Google Scholar] [CrossRef]
- Koch, M.; Blacque, O.; Venkatesan, K. Impact of 2,6-connectivity in azulene: Optical properties and stimuli responsive behavior. J. Mater. Chem. C 2013, 1, 7400–7408. [Google Scholar] [CrossRef]
- Ito, S.; Morita, N. Creation of stabilized electrochromic materials by taking advantage of azulene skeletons. Eur. J. Org. Chem. 2009, 27, 4567–4579. [Google Scholar] [CrossRef]
- Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C.J.; Robb, M.J. Modulating the Properties of Azulene-Containing Polymers through Controlled Incorporation of Regioisomers. Adv. Func. Mater. 2014, 24, 7338–7347. [Google Scholar] [CrossRef]
- Wang, X.; Ng, J.K.-P.; Jia, P.; Lin, T.; Cho, C.M.; Xu, J.; Lu, X.; He, C. Synthesis, electronic, and emission spectroscopy, and electrochromic characterization of azulene–fluorene conjugated oligomers and polymers. Macromolecules 2009, 42, 5534–5544. [Google Scholar] [CrossRef]
- Amir, E.; Amir, R.J.; Campos, L.M.; Hawker, C.J. Stimuli-responsive azulene-based conjugated oligomers with polyaniline-like properties. J. Am. Chem. Soc. 2011, 133, 10046–10049. [Google Scholar] [CrossRef]
- Razus, A.C. Azulene Moiety as Electron Reservoir in Positive Charged Systems; Short Survey. Symmetry 2021, 13, 526. [Google Scholar] [CrossRef]
- Lopez-Alled, C.M.; Park, S.J.; Lee, D.J.; Murfin, L.C.; Kociok-Kohn, G.; Hann, J.L.; Wenk, J.; James, T.D.; Kim, H.M.; Lewis, S.E. Azulene-based fluorescent chemosensor for adenosine diphosphate. Chem. Commun. 2021, 57, 10608–10611. [Google Scholar] [CrossRef]
- Dong, J.-X.; Zhang, H.-L. Azulene-based organic functional molecules for optoelectronics. Chin. Chem. Lett. 2016, 27, 1097–1104. [Google Scholar] [CrossRef]
- Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. Incorporation of 1,3-Free-2,6-Connected Azulene Units into the Back-bone of Conjugated Polymers: Improving Proton Responsiveness and Electrical Conductivity. ACS Macro Lett. 2019, 8, 1360–1364. [Google Scholar] [CrossRef]
- Herrmann, R.; Pedersen, B.; Wagner, G.; Youn, J.-H. Molecules with potential applications for non-linear optics: The combination of ferrocene and azulene. J. Organomet. Chem. 1998, 571, 261–266. [Google Scholar] [CrossRef]
- Iftime, G.; Lacroix, P.G.; Nakatani, K.; Razus, A.C. Push-pull azulene- based chromophores with nonlinear optical properties. Tetrahedron Lett. 1998, 39, 6853–6856. [Google Scholar] [CrossRef]
- Asato, A.E.; Liu, R.S.; Rao, V.P.; Cai, Y.M. Azulene-containing donor-acceptor compounds as second-order nonlinear chromophores. Tetrahedron Lett. 1996, 37, 419–422. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, P.; Ye, C.; Asato, A.E.; Liu, R.S. Theoretical investigation and molecular design of some azulene derivatives with large hyperpolarizabilities. J. Phys. Chem. A 1999, 103, 7076–7082. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Razus, A.C.; Buica, G.O.; Ungureanu, E.-M. Vinylazulenes chromophores: Synthesis and characterization. Dyes Pigm. 2016, 131, 246–255. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Arnold, G.-L.; Ungureanu, E.-M.; Razus, A.C. 1-Vinylazulenes-potential host molecules in ligands for metal ion detectors. Tetrahedron 2016, 72, 2316–2326. [Google Scholar] [CrossRef]
- Brotea, A.-G.; Matica, O.-T.; Musina (Borsaru), C.; Pandele, A.M.; Trusca, R.; Ungureanu, E.-M. Chemically Modified Electrodes Based on 4-((5-Isopropyl-3, 8-dimethylazulen-1-yl) methylene)-2-phenyloxazol-5 (4H)-one. Symmetry 2024, 16, 245. [Google Scholar] [CrossRef]
- Stefaniu, A.; Pop, M.-D.; Arnold, G.-L.; Birzan, L.; Pintilie, L.; Diacu, E.; Ungureanu, E.-M. DFT calculations and electrochemical studies on azulene ligands for heavy metal ions detection using chemically modified electrodes. J. Electrochem. Sci. Eng. 2018, 8, 73–85. [Google Scholar] [CrossRef]
- Ungureanu, E.-M.; Popescu (Apostoiu), M.; Tatu (Arnold), G.-L.; Birzan, L.; Isopescu, R.; Stanciu, G.; Buica, G.-O. Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones. Symmetry 2021, 13, 588. [Google Scholar] [CrossRef]
- Cristea, M.; Bîrzan, L.; Dumitrascu, F.; Enache, C.; Tecuceanu, V.; Hanganu, A.; Drăghici, C.; Deleanu, C.; Nicolescu, A.; Maganu, M.; et al. 1-Vinylazulenes with Oxazolonic Ring-Potential Ligands for Metal Ion Detectors; Synthesis and Products Properties. Symmetry 2021, 13, 1209. [Google Scholar] [CrossRef]
- Răducă, M.; Raţ, C.I.; Cristea, M. Crystal structure and Hirshfeld surface analysis of (Z)-2-Phenyl-4-((4,6,8-trimethylazulen-1-yl)methylene)oxazol-5(4H)-one. Rev. Roum. Chim. 2022, 67, 591–596. [Google Scholar] [CrossRef]
- Răducă, M.; Popa, M.M.; Cristea, M.; Razus, A.C.; Dumitrascu, F. Crystal structure and Hirshfeld surface analysis of (Z)-4-((5-isopropyl-3,8-dimethylazulen-1-yl)methylene)-2-phenyloxazol-5(4H)-one. Rev. Roum. Chim. 2025. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis Pro Software System; Rigaku Corporation: Oxford, UK, 2015; CrysAlis Pro v. 1.171.38.46. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]



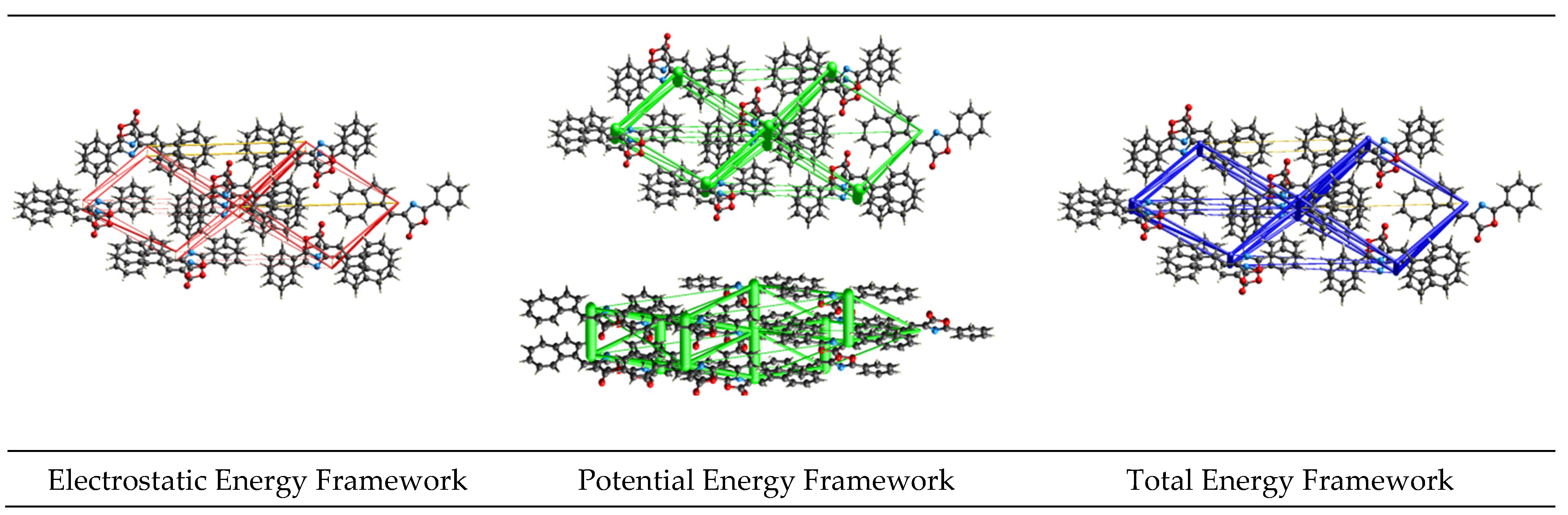
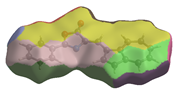
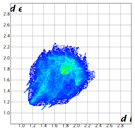 | 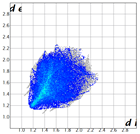 |  |  |  |
| All | H···H 43.5% | O···H 14.8% | C···H 20.6% | C···C 12.9% |
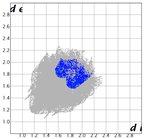 |  | 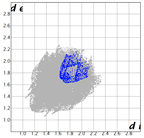 | 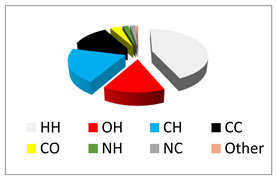 | |
| C···O 3.6% | N···H 2.1% | N···C 1.9% | ||
| dNorm | ShapeIndex | Curvedness | Fragment Patch |
|---|---|---|---|
 | 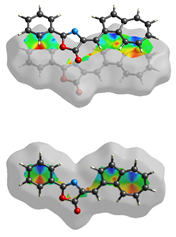 |  |  |
 |  | 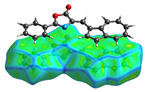 |  |
| Compound 3 | |
|---|---|
| Chemical formula | C20H13NO2 |
| M (g mol−1) | 299.31 |
| Temperature, (K) | 293(2) |
| Wavelength, (Å) | 1.54184 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 11.0744(3) |
| b (Å) | 3.87510(10) |
| c (Å) | 32.8947(7) |
| α (º) | 90 |
| β (º) | 92.045(2) |
| γ (º) | 90 |
| V (Å3) | 1410.76(6) |
| Z | 4 |
| Dc (g cm−3) | 1.409 |
| μ (mm−1) | 0.734 |
| F(000) | 624 |
| GOF | 1.014 |
| Final R1, wR2 [I > 2σ(I)] | 0.0400, 0.1020 |
| R1, wR2 (all data) | 0.0505, 0.1085 |
| Δρmin/Δρmax (e Å−3) | 0.20–0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristea, M.; Razus, A.C.; Shova, S.; Popa, M.-M.; Răducă, M.; Dumitrascu, F. (Z)-4-(Azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one. Molbank 2025, 2025, M2006. https://doi.org/10.3390/M2006
Cristea M, Razus AC, Shova S, Popa M-M, Răducă M, Dumitrascu F. (Z)-4-(Azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one. Molbank. 2025; 2025(2):M2006. https://doi.org/10.3390/M2006
Chicago/Turabian StyleCristea, Mihaela, Alexandru C. Razus, Sergiu Shova, Marcel-Mirel Popa, Mihai Răducă, and Florea Dumitrascu. 2025. "(Z)-4-(Azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one" Molbank 2025, no. 2: M2006. https://doi.org/10.3390/M2006
APA StyleCristea, M., Razus, A. C., Shova, S., Popa, M.-M., Răducă, M., & Dumitrascu, F. (2025). (Z)-4-(Azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one. Molbank, 2025(2), M2006. https://doi.org/10.3390/M2006








