5,7-Diiodoquinolin-8-yl (E)-3-(3,4-dihydroxyphenyl)acrylate
Abstract
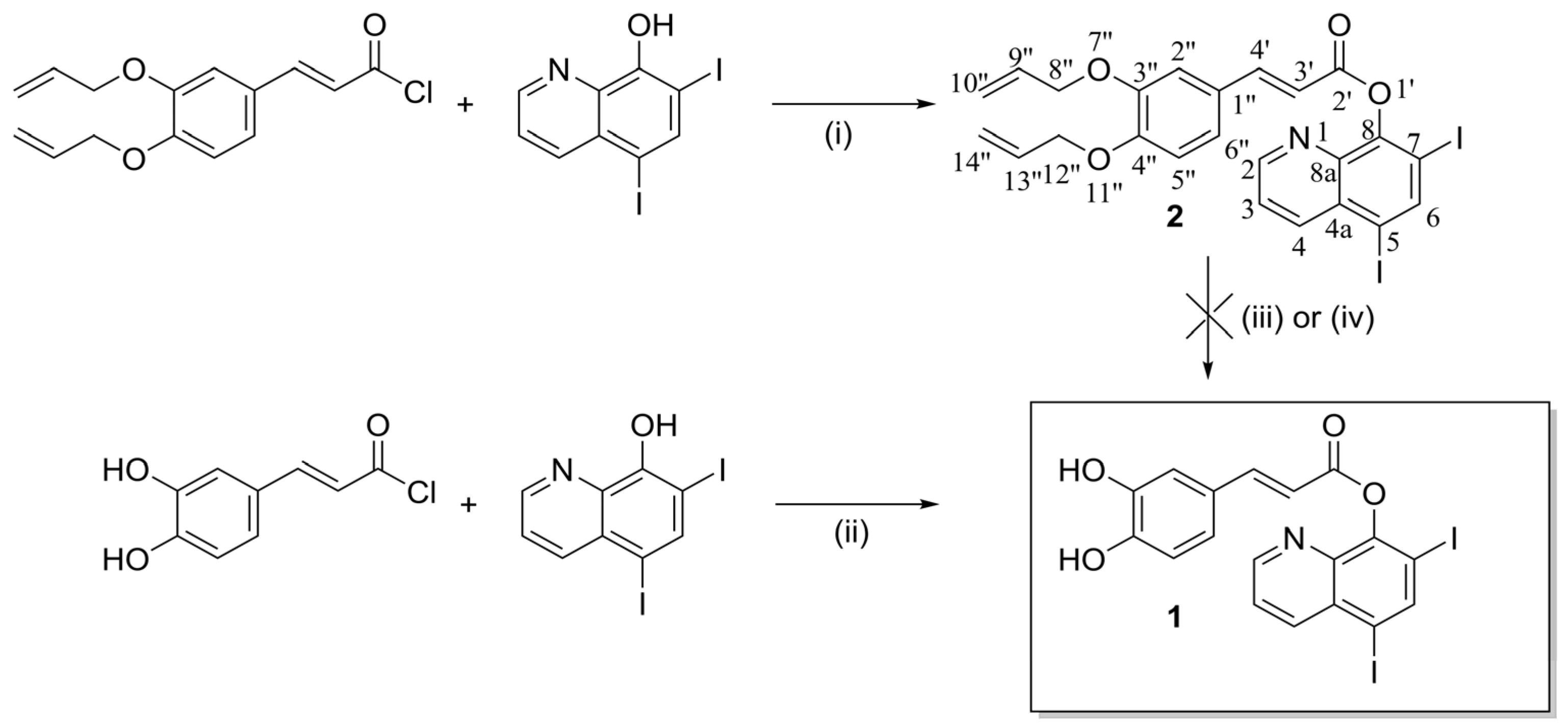
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 5,7-diiodoquinolin-8-yl (E)-3-(3,4-dihydroxyphenyl)acrylate (1)
3.2. Synthesis of 5,7-diiodoquinolin-8-yl (E)-3-(3,4-bis(allyloxy)phenyl)acrylate (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| ROS | Reactive oxygen species |
| DCM | Dichloromethane |
| DMF | N,N-Dimethylformamide |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| TEA | triethylamine |
| DMSO | Dimethylsufoxide |
| HRMS | High-resolution mass spectrometry |
| NMR | Nuclear magnetic resonance |
References
- Shirley, D.-A.T.; Farr, L.; Watanabe, K.; Moonah, S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Vila, T.; Rozental, S. Identification of two potential inhibitors of Sporothrix brasiliensis and Sporothrix schenckii in the Pathogen Box collection. PLoS ONE 2020, 15, e0240658. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, I.; Hafez, H.; AlZaim, I.; Tannous, C.; Ragab, H.; Hazzaa, A.; Ketat, S.; Ghoneim, A.; Katary, M.; Abd-Alhaseeb, M.M.; et al. Transforming iodoquinol into broad spectrum anti-tumor leads: Repurposing to modulate redox homeostasis. Bioorg. Chem. 2021, 113, 105035. [Google Scholar] [CrossRef] [PubMed]
- Abouelhassan, Y.; Garrison, A.T.; Burch, G.M.; Wong, W.; Norwood, V.M., IV.; Huigens, R.W., III. Discovery of quinoline small molecules with potent dispersal activity against methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis biofilms using a scaffold hopping strategy. Bioorg. Med. Chem. Lett. 2014, 24, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Aldaba-Muruato, L.R.; Ventura-Juárez, J.; Perez-Hernandez, A.M.; Hernández-Morales, A.; Muñoz-Ortega, M.H.; Martínez-Hernández, S.L.; Alvarado-Sánchez, B.; Macías-Pérez, J.R. Therapeutic Perspectives of p-Coumaric Acid: Anti-Necrotic, Anti-Cholestatic and Anti-Amoebic Activities. World Acad. Sci. J. 2021, 3, 47. [Google Scholar] [CrossRef]
- Jassim, A.F.; AL-Adilee, Y.M.S.; Mustafi, A.A. Role of Rosmarinus officinalis Phenolic Compounds in Treatment of Entamoeba histolytica Infection. Syst. Rev. Pharm. 2020, 11, 202–207. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, Y.; Chen, L.; Ge, M.; Yu, M.; Sun, Y.; Shen, L. Global Burden and Trends of the Entamoeba Infection-Associated Diseases from 1990 to 2019: An Observational Trend Study. Acta Trop. 2023, 240, 106866. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.M.S.; Herrmann, L.; Tsogoeva, S.B. Structural Hybridization as a Facile Approach to New Drug Candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Bukowski, N.; Pandey, J.L.; Doyle, L.; Richard, T.L.; Anderson, C.T.; Zhu, Y. Development of a Clickable Designer Monolignol for Interrogation of Lignification in Plant Cell Walls. Bioconjug. Chem. 2014, 25, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, M.; Sidoryk, K.; Zaremba-Czogalla, M.; Trzaskowski, B.; Kubiszewski, M.; Tobiasz, J.; Jaromin, A.; Michalak, O. The Conjugates of Indolo[2,3-b]quinoline as Anti-Pancreatic Cancer Agents: Design, Synthesis, Molecular Docking and Biological Evaluations. Int. J. Mol. Sci. 2024, 25, 2573. [Google Scholar] [CrossRef] [PubMed]
- Michalak, O.; Krzeczyński, P.; Cieślak, M.; Cmoch, P.; Cybulski, M.; Królewska-Golińska, K.; Kaźmierczak-Barańska, J.; Trzaskowski, B.; Ostrowska, K. Synthesis and Anti-Tumour, Immunomodulating Activity of Diosgenin and Tigogenin Conjugates. J. Steroid Biochem. Mol. Biol. 2020, 198, 105573. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Baran, P. Protecting-group-free synthesis as an opportunity for invention. Nature Chem. 2009, 1, 193. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Yu, C.-H.; Chang, Y.-P.; Lin, Y.-T.; Huang, C.-J.; Kuo, Y.-H.; Tsai, P.-J. Protective Effect of Caffeic Acid Derivatives on tert-Butyl Hydroperoxide-Induced Oxidative Hepato-Toxicity and Mitochondrial Dysfunction in HepG2 Cells. Molecules 2017, 22, 702. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cybulski, M.; Zieliński, M.; Kubiszewski, M.; Michalak, O. 5,7-Diiodoquinolin-8-yl (E)-3-(3,4-dihydroxyphenyl)acrylate. Molbank 2025, 2025, M2000. https://doi.org/10.3390/M2000
Cybulski M, Zieliński M, Kubiszewski M, Michalak O. 5,7-Diiodoquinolin-8-yl (E)-3-(3,4-dihydroxyphenyl)acrylate. Molbank. 2025; 2025(2):M2000. https://doi.org/10.3390/M2000
Chicago/Turabian StyleCybulski, Marcin, Michał Zieliński, Marek Kubiszewski, and Olga Michalak. 2025. "5,7-Diiodoquinolin-8-yl (E)-3-(3,4-dihydroxyphenyl)acrylate" Molbank 2025, no. 2: M2000. https://doi.org/10.3390/M2000
APA StyleCybulski, M., Zieliński, M., Kubiszewski, M., & Michalak, O. (2025). 5,7-Diiodoquinolin-8-yl (E)-3-(3,4-dihydroxyphenyl)acrylate. Molbank, 2025(2), M2000. https://doi.org/10.3390/M2000






