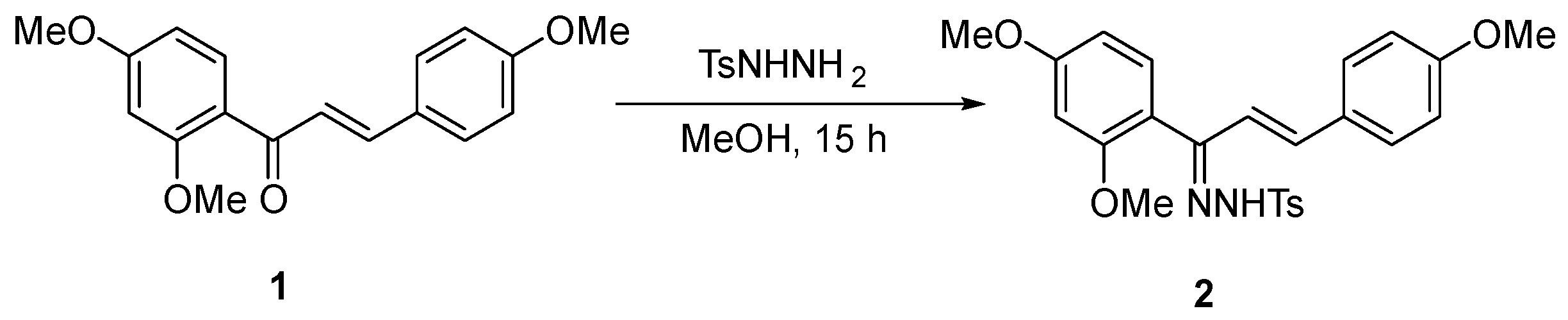
(E)-4,2′,4′-Trimethoxychalcone (Z)-N-Tosyl Hydrazone
Abstract
1. Introduction
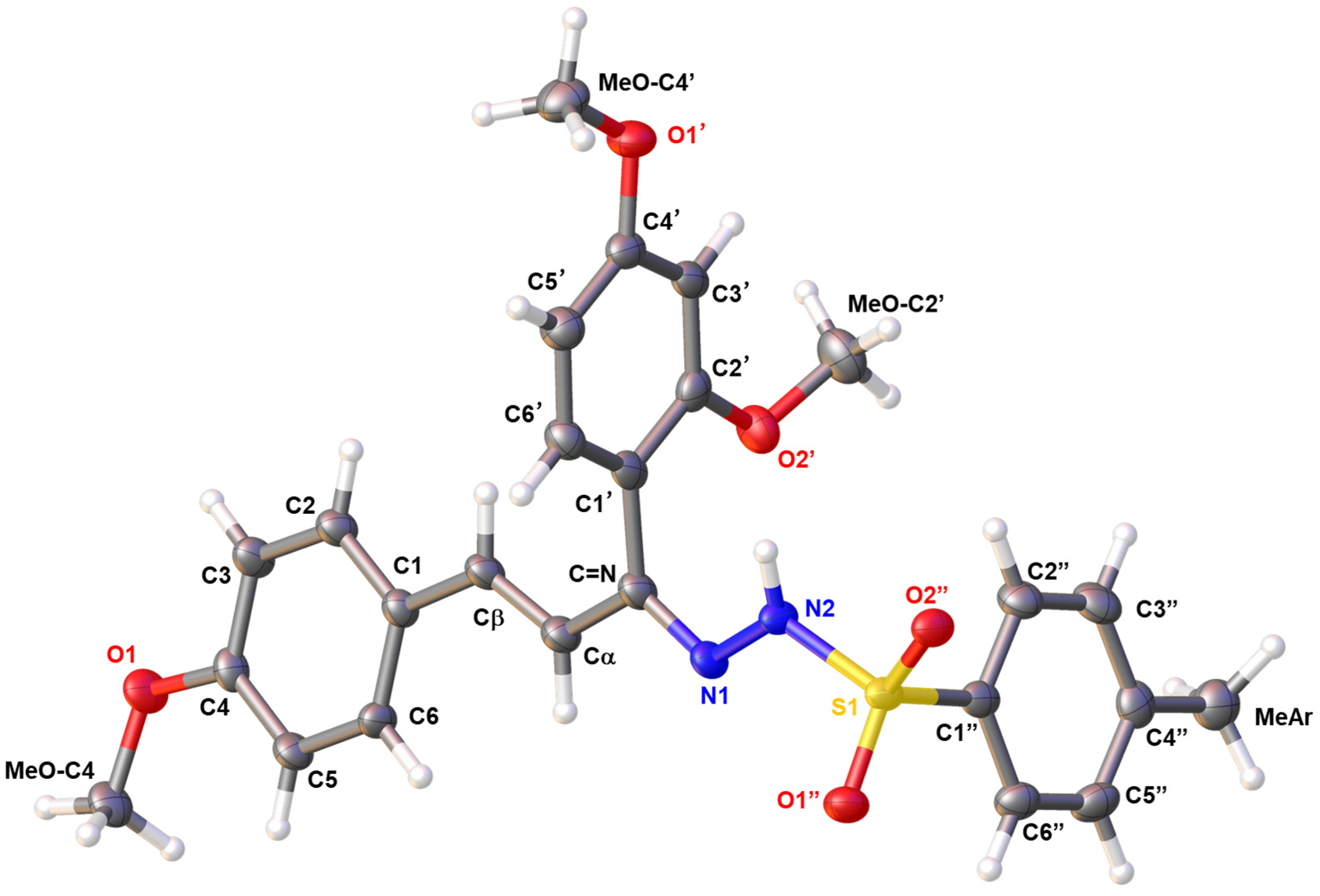
2. Results and Discussion
3. Materials and Methods
(E)-4,2′,4′-Trimethoxychalcone (Z)-N-Tosyl Hidrazone (2)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barluenga, J.; Valdés, C. Tosylhydrazones: New Uses for Classic Reagents in Palladium-Catalyzed Cross-Coupling and Metal-Free Reactions. Angew. Chem. Int. Ed. 2011, 50, 7486–7500. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, P.; Pan, Y.; Wang, N.; Bi, X. Who is Who in the Carbene Chemistry of N-Sulfonyl Hydrazones. Chin. J. Chem. 2024, 42, 2071–2108. [Google Scholar] [CrossRef]
- Bamford, W.R.; Stevens, T.S. The decomposition of toluene-p-sulphonylhydrazones by alkali. J. Chem. Soc. 1952, 4735–4740. [Google Scholar] [CrossRef]
- Caglioti, L. The reduction of tosylhydrazones and of acyl tosylhydrazides. Tetrahedron 1966, 22, 487–493. [Google Scholar] [CrossRef]
- Closs, G.L.; Closs, L.E.; Boll, W.A. The Base-Induced Pyrolysis of Tosylhydrazones of α,β-Unsaturated Aldehydes and Ketones. A Convenient Synthesis of Some Alkylcyclopropenes. J. Am. Chem. Soc. 1963, 85, 3796–3800. [Google Scholar] [CrossRef]
- Shapiro, R.H.; Duncan, J.H.; Clopton, J.C. An Investigation of the Intermediates in the Base-Catalyzed Decomposition of Camphor Tosylhydrazone in Aprotic Solvents. J. Am. Chem. Soc. 1967, 89, 471–472. [Google Scholar] [CrossRef]
- Eschenmoser, A.; Felix, D.; Ohloff, G. Eine neuartige Fragmentierung cyclischer α, ß-ungesättigter Carbonylsysteme; Synthese von Exalton und rac-Muscon aus Cyclododecanon Vorläufige Mitteilung. Helv. Chim. Acta 1967, 50, 708–713. [Google Scholar] [CrossRef]
- Tanabe, M.; Crowe, D.F.; Dehn, R.L.; Detre, G. The synthesis of secosteroid acetylenic ketones. Tetrahedron Lett. 1967, 8, 3739–3743. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Yang, D.T.C.; Baker, J.D. Deoxygenation of α,β-unsaturated aldehydes and ketones via the catecholborane reduction of the corresponding tosylhydrazones. J. Org. Chem. 1976, 41, 574–575. [Google Scholar] [CrossRef]
- Sinha, A.K.; Acharya, R.; Joshi, B.P. A Mild and Convenient Procedure for the Conversion of Toxic β-Asarone into Rare Phenylpropanoids: 2,4,5-Trimethoxycinnamaldehyde and γ-Asarone. J. Nat. Prod. 2002, 65, 764–765. [Google Scholar] [CrossRef]
- Shang, X.; Zhou, X.; Zhang, W.; Wan, C.; Chen, J. Tosylhydrazine mediated conjugate reduction and sequential reductive coupling cyclization: Synthesis of 2-arylchromans. Tetrahedron 2015, 71, 8187–8193. [Google Scholar] [CrossRef]
- Grandi, R.; Marchesini, A.; Pagnoni, U.M.; Trave, R. Conversion of conjugated p-tosylhydrazones to the corresponding ethers by sodium borohydride, sodium alkoxide, or potassium carbonate in alcohol solvents. J. Org. Chem. 1976, 41, 1755–1758. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J. N-Tosylhydrazones: Versatile synthons in the construction of cyclic compounds. Chem. Soc. Rev. 2017, 46, 2306–2362. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.; Gomez Pardo, D.; Tsuchiya, T.; Hillebrand, S.; Vors, J.-P.; Cossy, J. Modular, Concise, and Efficient Synthesis of Highly Functionalized 5-Fluoropyridazines by a [2 + 1]/[3 + 2]-Cycloaddition Sequence. Org. Lett. 2015, 17, 3414–3417. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Wu, J. AgOTf-catalyzed tandem reaction of N′-(2-alkynylbenzylidene)hydrazide with alkyne. Chem. Commun. 2009, 3469–3471. [Google Scholar] [CrossRef]
- Fu, X.; Li, H.; Ren, D.; Li, X. Synthesis of chromeno [3,4-c]pyrazole from N-tosylhydrazones and 3-nitro-2-phenyl-2H-chromene. J. Chem. Res. 2017, 41, 709–711. [Google Scholar] [CrossRef]
- Qiu, G.; Kuang, Y.; Wu, J. N-Imide Ylide-Based Reactions: C–H Functionalization, Nucleophilic Addition and Cycloaddition. Adv. Synth. Catal. 2014, 356, 3483–3504. [Google Scholar] [CrossRef]
- Fulton, J.R.; Aggarwal, V.K.; de Vicente, J. The Use of Tosylhydrazone Salts as a Safe Alternative for Handling Diazo Compounds and Their Applications in Organic Synthesis. Eur. J. Org. Chem. 2005, 2005, 1479–1492. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, H. N-Tosylhydrazones: Versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 2012, 41, 560–572. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Wang, J. Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions. Acc. Chem. Res. 2013, 46, 236–247. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Y.; Wang, J. Catalytic Cascade Reactions Involving Metal Carbene Migratory Insertion. ACS Catal. 2013, 3, 2586–2598. [Google Scholar] [CrossRef]
- López-Sánchez, C.; Álvarez-Corral, M.; Jiménez-González, L.; Muñoz-Dorado, M.; Rodríguez-García, I. Rh(II)-catalyzed enantioselective synthesis of acuminatin through a C–H insertion reaction of a non-stabilized carbenoid. Tetrahedron 2013, 69, 5511–5516. [Google Scholar] [CrossRef]
- Paveliev, S.A.; Segida, O.O.; Bityukov, O.V.; Tang, H.-T.; Pan, Y.-M.; Nikishin, G.I.; Terent’ev, A.O. Electrocatalytic Synthesis of Substituted Pyrazoles via Hypervalent Iodine Mediated Intramolecular C-N Coupling. Adv. Synth. Catal. 2022, 364, 3910–3916. [Google Scholar] [CrossRef]
- Mo, S.-K.; Teng, Q.-H.; Pan, Y.-M.; Tang, H.-T. Metal- and Oxidant-free Electrosynthesis of 1,2,3-Thiadiazoles from Element Sulfur and N-tosyl Hydrazones. Adv. Synth. Catal. 2019, 361, 1756–1760. [Google Scholar] [CrossRef]
- Korawat, H.S.; Saini, M.K.; Prajapati, K.; Singh, M.S.; Basak, A.K. One-Pot Expeditious Synthesis of Pyrazoloindolones via Base-Promoted Electrocyclization, C-N Coupling and Intramolecular Oxidative Cyclization. Synlett 2024, 35, 2385–2390. [Google Scholar] [CrossRef]
- Radolko, J.; Ehlers, P.; Langer, P. Recent Advances in Transition-Metal-Catalyzed Reactions of N-Tosylhydrazones. Adv. Synth. Catal. 2021, 363, 3616–3654. [Google Scholar] [CrossRef]
- Qiu, D.; Mo, F.; Zhang, Y.; Wang, J. Chapter Two—Recent Advances in Transition-Metal-Catalyzed Cross-Coupling Reactions With N-Tosylhydrazones. Adv. Organomet. Chem. 2017, 67, 151–219. [Google Scholar] [CrossRef]
- Singhal, R.; Choudhary, S.P.; Malik, B.; Pilania, M. Emerging Trends in N-Tosylhydrazone Mediated Transition-Metal-Free Reactions. ChemistrySelect 2022, 7, e202200134. [Google Scholar] [CrossRef]
- Arunprasath, D.; DeviBala, B.; Sekar, G. Luxury of N-Tosylhydrazones in Transition-Metal-Free Transformations. Adv. Synth. Catal. 2019, 361, 1172–1207. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, H.; Fu, T.; Xia, Y.; Qiu, D.; Zhang, Y.; Wang, J. Pd(0)-Catalyzed Carbene Insertion into Si–Si and Sn–Sn Bonds. J. Am. Chem. Soc. 2015, 137, 12800–12803. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Wang, X.; Yu, Y.; Zheng, J.; Wu, W.; Jiang, H. Copper-catalyzed cyanothiolation to incorporate a sulfur-substituted quaternary carbon center. Chem. Sci. 2017, 8, 7047–7051. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Maqueda, A.; Lopez, L.; Plaza, M.; Valdes, C. Synthesis of substituted benzylboronates by light promoted homologation of boronic acids with N-sulfonylhydrazones. ChemRxiv 2023, 14, 13765–13775. [Google Scholar] [CrossRef]
- de Gracia Retamosa, M.; Matador, E.; Monge, D.; Lassaletta, J.M.; Fernández, R. Hydrazones as Singular Reagents in Asymmetric Organocatalysis. Chem. Eur. J. 2016, 22, 13430–13445. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ma, M.; Zhang, Q.; Luo, H.; Wang, T.; Chai, Y. Metal-Free Synthesis of Pyrazoles from 1,3-Diarylpropenes and Hydrazines via Multiple Inter-/Intramolecular C-H Aminations. Adv. Synth. Catal. 2017, 359, 2610–2620. [Google Scholar] [CrossRef]
- Grandi, R.; Messerotti, W.; Pagnoni, U.M.; Trave, R. Decomposition of conjugated p-tosylhydrazones in base. Partition between solvolysis and cycloaddition products. J. Org. Chem. 1977, 42, 1352–1355. [Google Scholar] [CrossRef]
- Pecnard, S.; Provot, O.; Levaique, H.; Bignon, J.; Askenatzis, L.; Saller, F.; Borgel, D.; Michallet, S.; Laisne, M.-C.; Lafanechere, L.; et al. Cyclic bridged analogs of isoCA-4: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 209, 112873. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, D.; Mishra, B.K.; Tiwari, B. Iodobenzene-Catalyzed Synthesis of Fully Functionalized NH-Pyrazoles and Isoxazoles from α,β-Unsaturated Hydrazones and Oximes via 1,2-Aryl Shift. Org. Lett. 2024, 26, 385–389. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Z.; Yang, X.; Wu, J. Tandem Reactions of N′-(2-Alkynylbenzylidene)hydrazides with Silyl Enolates: A Facile Route to H-Pyrazolo [5,1-a]isoquinolines. J. Comb. Chem. 2010, 12, 374–378. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, S.; Álvarez-Corral, M.; Jiménez-González, L.; López-Sánchez, C.; Rosales, A.; Muñoz-Dorado, M.; Rodríguez-García, I. Synthesis of dihydrodehydrodiconiferyl alcohol and derivatives through intramolecular C–H insertion. Tetrahedron 2006, 62, 12182–12190. [Google Scholar] [CrossRef]
- Rosales, A.; Rodríguez-García, I.; López-Sánchez, C.; Álvarez-Corral, M.; Muñoz-Dorado, M. Solvent influence in the Rh-catalyzed intramolecular 1,6 C–H insertions: A general approach to the chromane and flavanone skeletons. Tetrahedron 2011, 67, 3071–3075. [Google Scholar] [CrossRef]
- López-Sánchez, C.; Álvarez-Corral, M.; Muñoz-Dorado, M.; Rodríguez-García, I. Efficient Intramolecular C–H Insertion Catalyzed by Iridium Porphyrin Complexes. Synlett 2012, 23, 2469–2472. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Crespi, E.J.; Pedersen, J.Z.; Nakano, G.; Duong, M.; McKee, C.; Lee, S.; Jiwrajka, M.; Caldwell, C.; et al. Probing antioxidant activity of 2′-hydroxychalcones: Crystal and molecular structures, in vitro antiproliferative studies and in vivo effects on glucose regulation. Biochimie 2013, 95, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Blatova, O.A.; Asiri, A.M.; Al-amshany, Z.M.; Arshad, M.N.; Blatov, V.A. Molecular packings and specific-bonding patterns in sulfonamides. New J. Chem. 2014, 38, 4099–4106. [Google Scholar] [CrossRef]
- Krohn, K.; Ahmed, I.; John, M. Enantioselective Synthesis of Flavan-3-ols Using a Mitsunobu Cyclization. Synthesis 2009, 2009, 779–786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenguel Gómez, S.; Moreno-Gutiérrez, I.; Muñoz-Dorado, M.; Álvarez-Corral, M.; Rodríguez-García, I. (E)-4,2′,4′-Trimethoxychalcone (Z)-N-Tosyl Hydrazone. Molbank 2025, 2025, M1997. https://doi.org/10.3390/M1997
Berenguel Gómez S, Moreno-Gutiérrez I, Muñoz-Dorado M, Álvarez-Corral M, Rodríguez-García I. (E)-4,2′,4′-Trimethoxychalcone (Z)-N-Tosyl Hydrazone. Molbank. 2025; 2025(2):M1997. https://doi.org/10.3390/M1997
Chicago/Turabian StyleBerenguel Gómez, Sonia, Irene Moreno-Gutiérrez, Manuel Muñoz-Dorado, Míriam Álvarez-Corral, and Ignacio Rodríguez-García. 2025. "(E)-4,2′,4′-Trimethoxychalcone (Z)-N-Tosyl Hydrazone" Molbank 2025, no. 2: M1997. https://doi.org/10.3390/M1997
APA StyleBerenguel Gómez, S., Moreno-Gutiérrez, I., Muñoz-Dorado, M., Álvarez-Corral, M., & Rodríguez-García, I. (2025). (E)-4,2′,4′-Trimethoxychalcone (Z)-N-Tosyl Hydrazone. Molbank, 2025(2), M1997. https://doi.org/10.3390/M1997





