2,4,6-Trichloro-cyclohexa-2,5-dienone
Abstract
1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leftwick, A.P.; Parnell, E.W. New Pesticides Derived from Cyclohexadiene, OA5232A, Application Date 1976, Publication Date 1981. Available online: https://patents.google.com/patent/OA05232A/en?q=(A.P)&inventor=Leftwick%2c&oq=Leftwick%2c+A.P (accessed on 14 February 2025).
- Takegawa, B. Steroid Compound, JPH0267296A. 1988. Available online: https://patents.google.com/patent/JPH0267296A/en?oq=JPH0267296 (accessed on 14 February 2025).
- Mukawa, F. 10β-Chloro-17β-hydroxyestra-1,4-dien-3-one and its related compounds. J. Chem. Soc. Perkin Trans. 1 1988, 457–460. [Google Scholar] [CrossRef]
- Kaiser, K.L.E.; Niculescu, S.P. On the PNN modeling of estrogen receptor binding data for carboxylic acid esters and organochlorine compounds. Water Qual. Res. J. Can. 2001, 36, 619–630. [Google Scholar] [CrossRef]
- Guy, A.; Lemaire, M.; Guetté, J.P. Halogenation regioselective en serie aromatique-II. Chloration des naphtols et de leurs ethers a l’aide de reactifs mettant en jeu des interactions du type donneur-accepteur. Tetrahedron 1982, 38, 2347–2354. [Google Scholar] [CrossRef]
- Mamaghani, M.; Zolfigol, M.A.; Shojaei, M. N-Chloro-2,3,4,4,5,6-hexachlorocyclohexa-2,5-dienylideneamine as a mild and highly regioselective chlorinating reagent. Synth. Commun. 2002, 32, 735–740. [Google Scholar] [CrossRef]
- Fischer, A.; Henderson, G.N. Ipso chlorination of 4-alkylphenols. Formation of 4-alkyl-4-chlorocyclohexa-2,5-dienones. Can. J. Chem. 1979, 57, 552–557. [Google Scholar] [CrossRef]
- Bergquist, K.-E.; Nilsson, A.; Ronlán, A.; Norin, T.; Hjeds, H. Electrophilic Chlorination of 4-Methylphenols with Molecular Chlorine. Synthesis of Dimethoxy Aromatics by Methanolysis of 4-Chloro-4-methylcyclohexa-2,5-dienones. Acta Chem. Scand. 1982, 36, 675–683. [Google Scholar] [CrossRef][Green Version]
- Antinori, G.; Baciocchi, E.; Illuminati, G. Non-conventional paths in electrophilic aromatic reactions. Part VI. Chlorination of 3,5-dichloro-2,4,6-trimethylanisole and related compounds. J. Chem. Soc. B Phys. Org. 1969, 373–377. [Google Scholar] [CrossRef]
- Benedikt, R.; Schmidt, M.V. Notizen über Halogenderivate. Monatshefte Für Chem. 1983, 4, 604–609. [Google Scholar] [CrossRef]
- Mukawa, F.; Suzuki, T.; Ishibashi, M.; Yamada, F. Estrogen and androgen receptor binding affinity of 10β-chloro-estrenen derivatives. J. Steroid Biochem. 1988, 31, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Mukawa, F. The anomalous chlorination of estradiol 17β-acetate with isocyanuric chloride. Tetrahedron Lett. 1959, 1, 17–20. [Google Scholar] [CrossRef]
- Ferron, B.; Jacquesy, J.C.; Jouannetaud, M.P.; Karam, O.; Coustard, J.M. Ipso-chlorination of 4-alkylphenols ethers a novel route to 4-chlorocyclohexa-2,5-dienones. Tetrahedron Lett. 1993, 34, 2949–2952. [Google Scholar] [CrossRef]
- Uyanik, M.; Sasakura, N.; Kuwahata, M.; Ejima, Y.; Ishihara, K. Practical oxidative dearomatization of phenols with sodium hypochlorite pentahydrate. Chem. Lett. 2015, 44, 381–383. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Xu, D.; Xia, C.; Sun, W. Direct halogenative dearomatization of 2-naphthols by NXS (X = Cl, Br) in water. Green Chem. 2016, 18, 5485–5492. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, S.G.; Liang, X.W.; Gao, D.W.; Zheng, J.; You, S.L. Organocatalytic asymmetric chlorinative dearomatization of naphthols. Chem. Sci. 2015, 6, 4179–4183. [Google Scholar] [CrossRef]
- Uyanik, M.; Sahara, N.; Ishihara, K. Regioselective Oxidative Chlorination of Arenols Using NaCl and Oxone. Euro. J. Org. Chem. 2019, 2019, 27–31. [Google Scholar] [CrossRef]
- Tilstam, U.; Weinmann, H. Trichloroisocyanuric Acid: A Safe and Efficient Oxidant. Org. Process Res. Dev. 2002, 6, 384–393. [Google Scholar] [CrossRef]
- Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L. Trichloroisocyanuric Acid: A Versatile and Efficient Chlorinating and Oxidizing Reagent. Euro. J. Org. Chem. 2019, 2019, 3544–3552. [Google Scholar] [CrossRef]
- Gambacorta, G.; Sharley, J.S.; Baxendale, I.R. A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries. Beilstein J. Org. Chem. 2021, 17, 1181–1312. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, P.; Williams, J.D.; Hone, C.A.; Kappe, C.O. Laboratory of the future: A modular flow platform with multiple integrated PAT tools for multistep reactions. React. Chem. Eng. 2019, 4, 1571–1578. [Google Scholar] [CrossRef]
- Webb, D.; Jamison, T.F. Continuous flow multi-step organic synthesis. Chem. Sci. 2010, 1, 675–680. [Google Scholar] [CrossRef]
- Pastre, J.C.; Browne, D.L.; Ley, S.V. Flow chemistry syntheses of natural products. Chem. Soc. Rev. 2013, 42, 8849–8869. [Google Scholar] [CrossRef]
- Sasaki, T.; Yahata, K.; Isomura, M.; Ohashi, I.; Fukuyama, T.; Miyashita, Y.; Watanabe, Y.; Murai, N.; Matsuda, M.; Kamada, A.; et al. What Does It Take to Develop Structurally Complex Molecules by Total Synthesis? Rapid Process Development and GMP Manufacturing of E7130 Drug Substance for First-in-Human Clinical Study. Org. Process Res. Dev. 2024, 28, 2077–2089. [Google Scholar] [CrossRef]
- Browne, D.L.; Deadman, B.J.; Ashe, R.; Baxendale, I.R.; Ley, S.V. Continuous Flow Processing of Slurries: Evaluation of an Agitated Cell Reactor. Org. Process Res. Dev. 2011, 15, 693–697. [Google Scholar] [CrossRef]
- Deadman, B.J.; Browne, D.L.; Baxendale, I.R.; Ley, S.V. Back Pressure Regulation of Slurry-Forming Reactions in Continuous Flow. Chem. Eng. Technol. 2015, 38, 259–264. [Google Scholar] [CrossRef]
- Sharma, M.K.; Suru, A.; Joshi, A.; Kulkarni, A.A. A Novel Flow Reactor for Handling Suspensions: Hydrodynamics and Performance Evaluation. Ind. Eng. Chem. Res. 2020, 59, 16462–16472. [Google Scholar] [CrossRef]
- Bianchi, P.; Williams, J.D.; Kappe, C.O. Oscillatory flow reactors for synthetic chemistry applications. J. Flow Chem. 2020, 10, 475–490. [Google Scholar] [CrossRef]
- Doyle, B.J.; Gutmann, B.; Bittel, M.; Hubler, T.; Macchi, A.; Roberge, D.M. Handling of Solids and Flow Characterization in a Baffleless Oscillatory Flow Coil Reactor. Ind. Eng. Chem. Res. 2020, 59, 4007–4019. [Google Scholar] [CrossRef]
- Hartman, R.L.; Naber, J.R.; Zaborenko, N.; Buchwald, S.L.; Jensen, K.F. Overcoming the Challenges of Solid Bridging and Constriction during Pd-Catalyzed C−N Bond Formation in Microreactors. Org. Process Res. Dev. 2010, 14, 1347–1357. [Google Scholar] [CrossRef]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S. Fouling in microstructured devices: A review. Chem. Commun. 2015, 51, 8213–8228. [Google Scholar] [CrossRef]
- Wu, K.; Kuhn, S. Strategies for solids handling in microreactors. Chim. Oggi/Chem. Today 2014, 32, 62–66. [Google Scholar]
- Dong, Z.; Zhao, S.; Zhang, Y.; Yao, C.; Yuan, Q.; Chen, G. Mixing and residence time distribution in ultrasonic microreactors. AIChE J. 2017, 63, 1404–1418. [Google Scholar] [CrossRef]
- Rivas, D.F.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Delacour, C.; Carogher, K.M.; Udepurkar, A.P.; Kuhn, S. Continuous ultrasonic reactors: Design, mechanism and application. Materials 2020, 13, 344. [Google Scholar] [CrossRef]
- Chip, G.K.; Grossert, J.S. Aromatic halogenation with titanium (IV) chloride in presence of peroxytrifluoroacetic acid. Can. J. Chem. 1972, 50, 1233–1240. [Google Scholar] [CrossRef]
- Husain, S.; Kifayatullah, M. Phenol-dienone rearrangement in the chlorination of p-cresol with tert-butyl hypochlorite. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1985, 24B, 711–714. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]

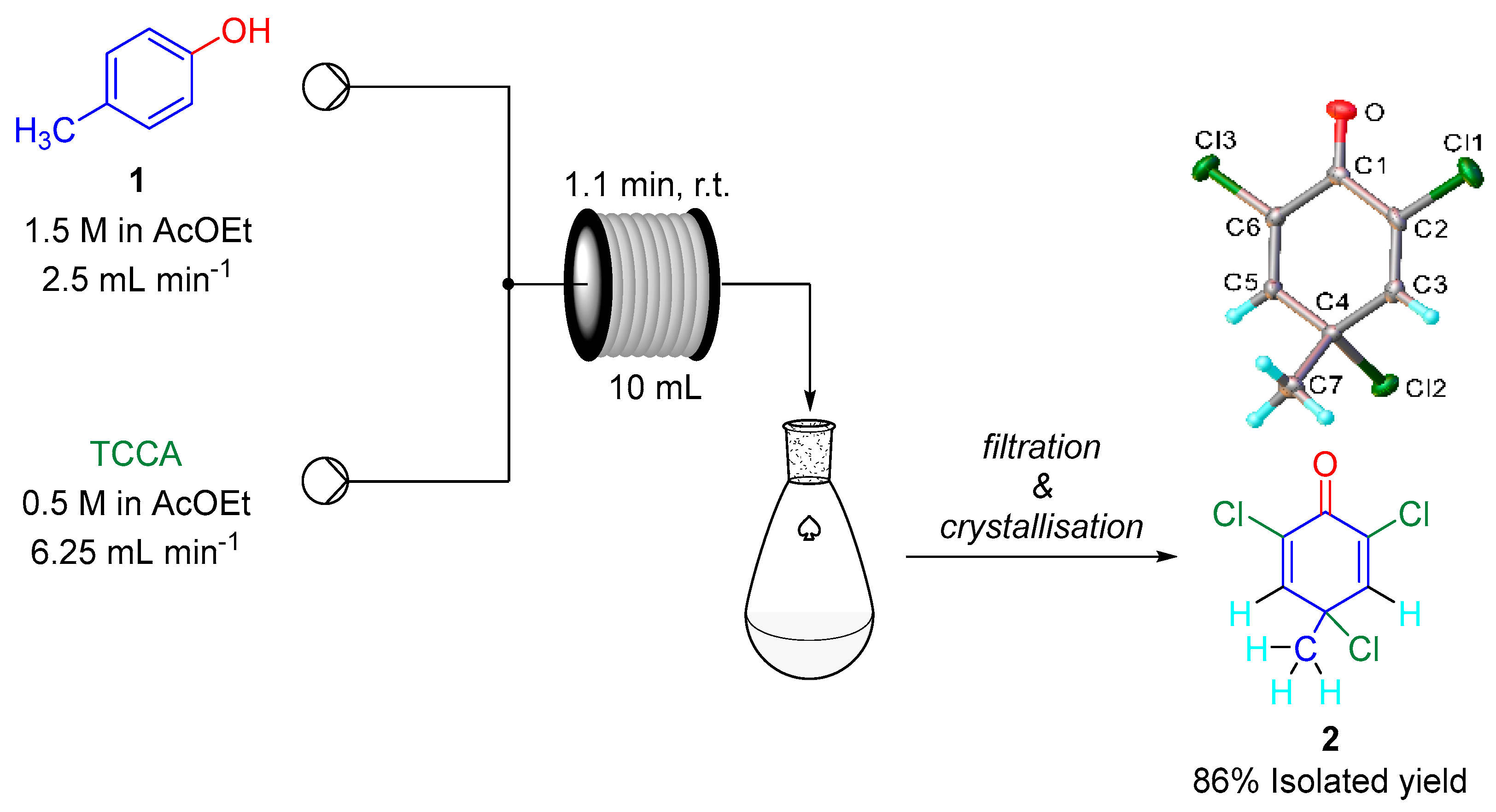

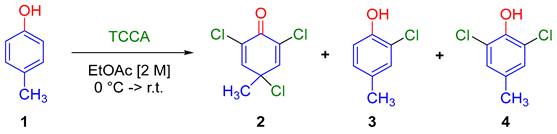 | |||||
| Entry 1 | TCCA (equiv.) | Residence Time (min) | 2 | 3 | 4 |
| 1 | 2 | 20 | 57.7 | 42.3 | - |
| 2 | 2 | 30 | 75.0 | 25.0 | - |
| 3 | 2 | 60 | 67.0 | 20.9 | 11.9 |
| 4 | 2.2 | 30 | 75.0 | 25.0 | - |
| 5 | 2.5 | 30 | 84.9 | 9.4 | 5.7 |
| 6 | 2.7 | 30 | 83.7 | 16.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambacorta, G.; Teo, Q.H.; Baxendale, I.R. 2,4,6-Trichloro-cyclohexa-2,5-dienone. Molbank 2025, 2025, M1969. https://doi.org/10.3390/M1969
Gambacorta G, Teo QH, Baxendale IR. 2,4,6-Trichloro-cyclohexa-2,5-dienone. Molbank. 2025; 2025(1):M1969. https://doi.org/10.3390/M1969
Chicago/Turabian StyleGambacorta, Guido, Qin Han Teo, and Ian R. Baxendale. 2025. "2,4,6-Trichloro-cyclohexa-2,5-dienone" Molbank 2025, no. 1: M1969. https://doi.org/10.3390/M1969
APA StyleGambacorta, G., Teo, Q. H., & Baxendale, I. R. (2025). 2,4,6-Trichloro-cyclohexa-2,5-dienone. Molbank, 2025(1), M1969. https://doi.org/10.3390/M1969







