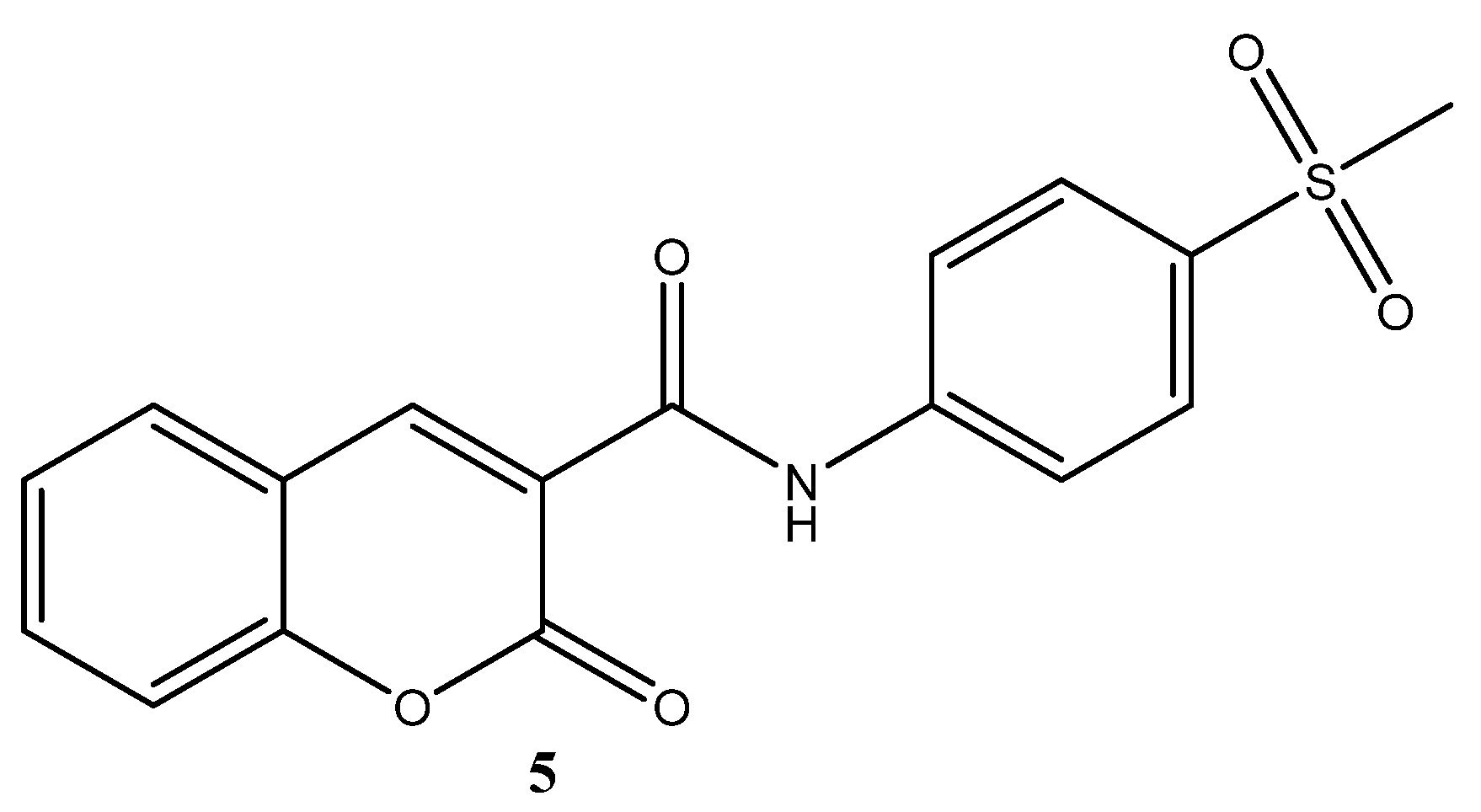
N-(4-Methoxyphenethyl)-2-oxo-2H-chromene-3-carboxamide
Abstract
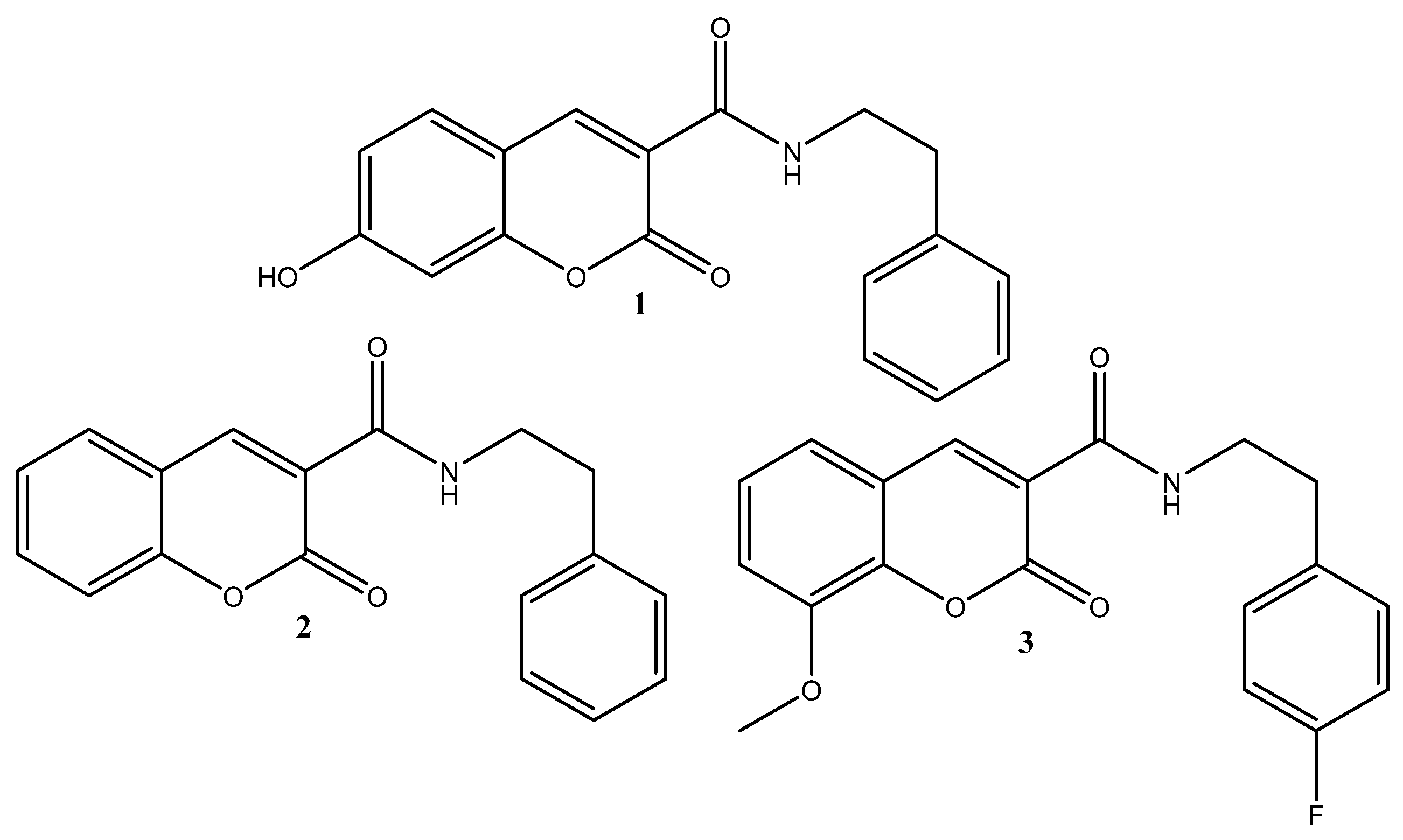
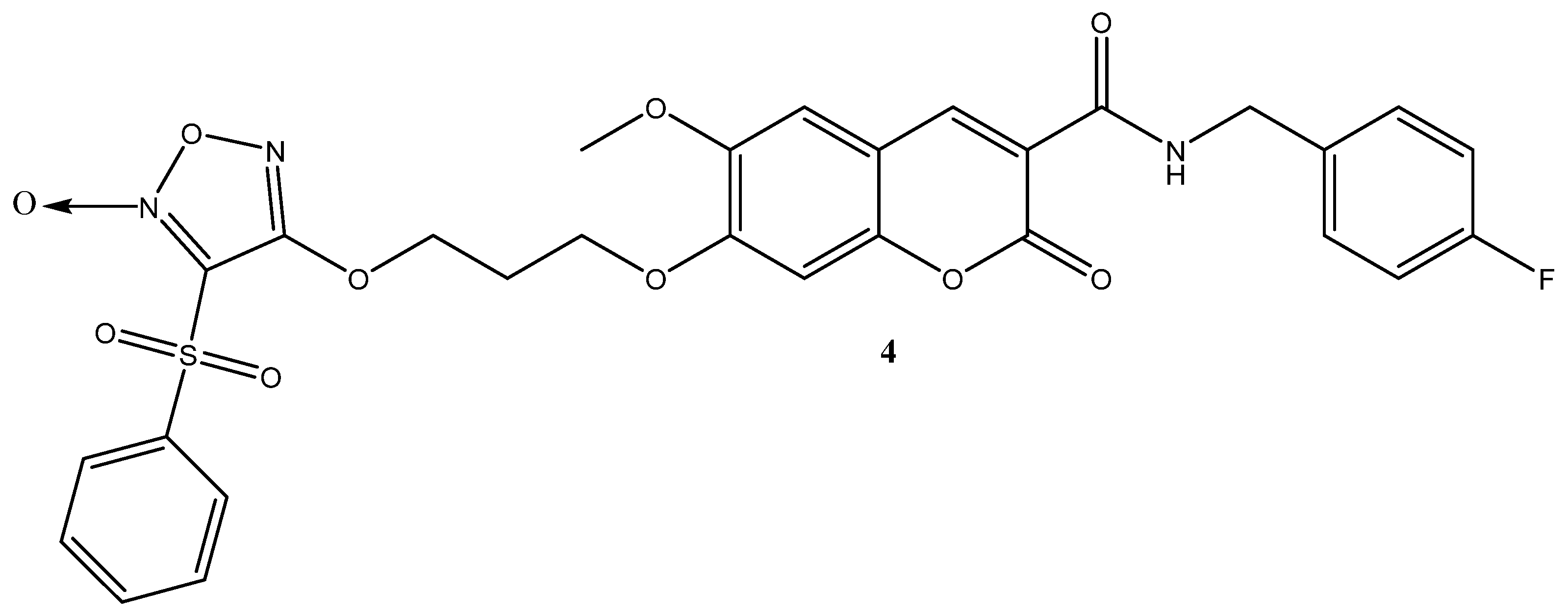
1. Introduction
2. Results and Discussion
3. Materials and Methods
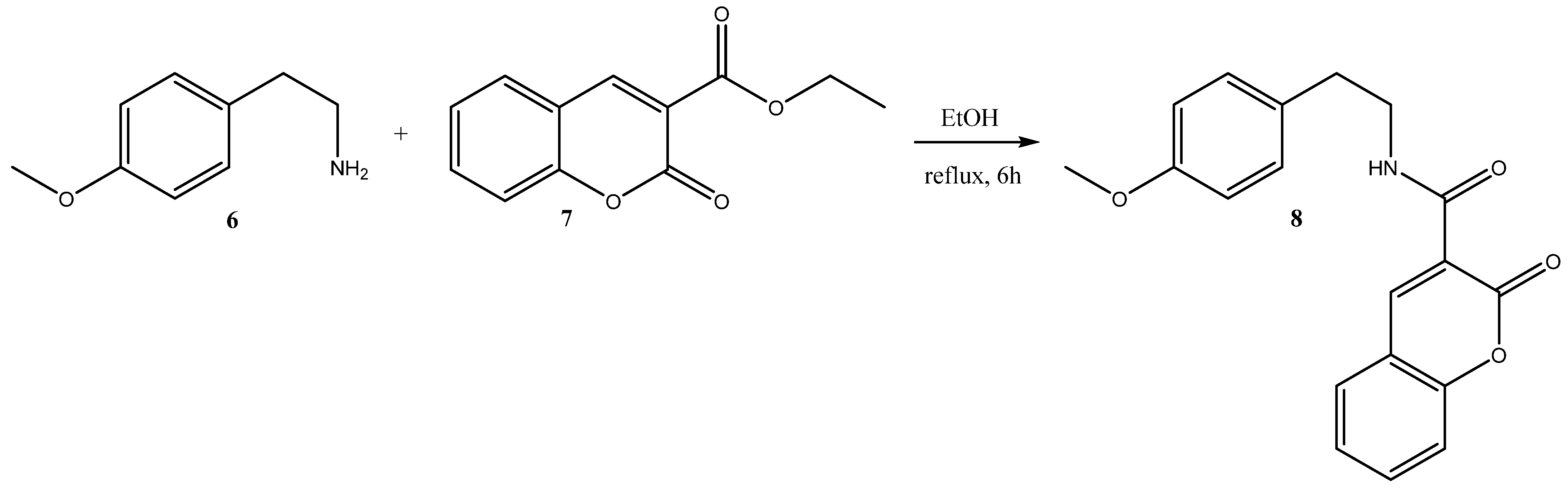
3.1. Synthetic Procedures
3.1.1. Synthesis of Ethyl 2-Oxo-2H-chromene-3-carboxylate 7
3.1.2. Synthesis of N-(4-methoxyphenethyl)-2-oxo-2H-chromene-3-carboxamide 8
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, M.K.; Khatoon, S.; Khan, M.F.; Akhtar, M.S.; Ahamad, S.; Saquib, M. Coumarins as versatile therapeutic phytomolecules: A systematic review. Phytomedicine 2024, 134, 155972. [Google Scholar] [CrossRef] [PubMed]
- Ramsis, T.M.; Ebrahim, M.A.; Fayed, E.A. Synthetic coumarin derivatives with anticoagulation and antiplatelet aggregation inhibitory effects. Med. Chem. Res. 2023, 32, 2269–2278. [Google Scholar] [CrossRef]
- Rostom, B.; Karaky, R.; Kassab, I.; Veitía, M.S.-I. Coumarins derivatives and inflammation: Review of their effects on the inflammatory signaling pathways. Eur. J. Pharmacol. 2022, 922, 174867. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Koley, M.; Han, J.; Soloshonok, V.A.; Mojumder, S.; Javahershenas, R.; Makarem, A. Latest developments in coumarin-based anticancer agents: Mechanism of action and structure–activity relationship studies. RSC Med. Chem. 2024, 15, 10–54. [Google Scholar] [CrossRef] [PubMed]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Buzjak Služek, V.B.; Cindrić, I.; Molnar, M. Coumarins in Food and Methods of Their Determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Okada, T.; Dibwe, D.; Maruyama, T.; Takahara, S.; Okada, T.; Endo, S.; Tayooka, N. Design and synthesis of functionalized coumarins as potential anti-austerity agents that eliminates cancer cells’ tolerance to nutrition starvation. Bioorganic Med. Chem. Lett. 2019, 29, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Das, A.; Jangid, K.; Kumar, A.; Kumar, V.; Jaitak, V. Identification of coumarin derivatives targeting acetylcholinesterase for Alzheimer’s disease by field-based 3D-QSAR, pharmacophore model-based virtual screening, molecular docking, MM/GBSA, ADME and MD simulation study. Curr. Res. Struct. Biol. 2024, 7, 100124. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, N.; Wang, K.; Deng, Q.; Lei, Z.; Sun, J.; Chen, L. Design, synthesis and biological activity evaluation of novel scopoletin-NO donor derivatives against MCF-7 human breast cancer in vitro and in vivo. Eur. J. Med. Chem. 2021, 224, 113701. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Bizzarri, B.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; et al. Synthesis, Molecular Modeling, and Selective Inhibitory Activity against Human Monoamine Oxidases of 3-Carboxamido-7-Substituted Coumarins. J. Med. Chem. 2009, 52, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, F.J.; Padilla-Martínez, I.I.; Trujillo-Ferrara, J. Spectral assignments and reference data. Magn. Reson. Chem. 2001, 39, 765–767. [Google Scholar] [CrossRef]
- Liu, K.; Song, F.; Wang, J.; Wang, X.; Kan, C. A V-shaped bis-coumarin based fluorescence probe for F- detection in tea infusions and potable water and bioimaging applications in living systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 316, 124349. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Huang, C.; Zhang, J.; Zhong, L.; Zeng, R.; Kuang, Y.; Ye, X.; Xie, Z.; Zhang, J.; Wang, Z.; et al. Synthesis, bioactivity and molecular docking of novel coumarin-quinolinamide containing monocyclic monoterpenes as potential SDH inhibitors. J. Mol. Struct. 2024, 1315, 138785. [Google Scholar] [CrossRef]
- Moawad, S.S.; Abou-El-Regal, M.M.; El-Metwally, S.A. Design, synthesis and biological assessment of novel pyridopyrimidine and chromenopyridopyrimidine analogues. Synth. Commun. 2025, 55, 65–75. [Google Scholar] [CrossRef]
- Saini, K.; Raigar, A.K.; Manju; Jyoti, N.; Guleria, A. Sustainable aqueous-mediated one-pot synthesis of 2-hydroxy-5-oxo-5,6,7,8-tetrahydro-4H-chromene and coumarin derivatives using taurine as the green bioorganic catalyst. Tetrahedron 2024, 168, 134346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, I.; Manolov, S.; Dimitrova, D.; Nedialkov, P. N-(4-Methoxyphenethyl)-2-oxo-2H-chromene-3-carboxamide. Molbank 2025, 2025, M1968. https://doi.org/10.3390/M1968
Ivanov I, Manolov S, Dimitrova D, Nedialkov P. N-(4-Methoxyphenethyl)-2-oxo-2H-chromene-3-carboxamide. Molbank. 2025; 2025(1):M1968. https://doi.org/10.3390/M1968
Chicago/Turabian StyleIvanov, Iliyan, Stanimir Manolov, Diyana Dimitrova, and Paraskev Nedialkov. 2025. "N-(4-Methoxyphenethyl)-2-oxo-2H-chromene-3-carboxamide" Molbank 2025, no. 1: M1968. https://doi.org/10.3390/M1968
APA StyleIvanov, I., Manolov, S., Dimitrova, D., & Nedialkov, P. (2025). N-(4-Methoxyphenethyl)-2-oxo-2H-chromene-3-carboxamide. Molbank, 2025(1), M1968. https://doi.org/10.3390/M1968







