Thorium(IV) and Uranium(IV) Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea) Ligands
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Radiation Precautions
3.3. Synthesis of [Th(2,6-dipicolinate)2(H2O)4] (1)
3.4. Synthesis of (NH4)2[Th(Lpic)3] (2)
3.5. Synthesis of [UAu2(Lpic)3] (3)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyer, L.; Hoyer, E.; Hennig, H.; Kirmse, R.; Hartmann, H.; Liebscher, J. Synthese und Charakterisierung neuartiger Übergangsmetallchelate von 1,1-Dialkyl-3-benzoyl-thioharnstoffen. J. Prakt. Chem. 1975, 317, 829–839. [Google Scholar] [CrossRef]
- Beyer, L.; Hoyer, E.; Liebscher, J.; Hartmann, H. Komplexbildung mit N-Acyl-thioharnstoffen. Z. Chem. 1981, 21, 81–91. [Google Scholar] [CrossRef]
- Koch, K.R. New chemistry with old ligands: N-alkyl- and N, N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord. Chem. Rev. 2001, 216−217, 473–488. [Google Scholar] [CrossRef]
- Cambridge Crystallographic Database, Version 5.44. Available online: https://www.ccdc.cam.ac.uk (accessed on 1 December 2024).
- Koch, K.R.; Bourne, S.A.; Coetzee, A.; Miller, J. Self-assembly of 2:2 and 3:3 metallamacrocyclic complexes of platinum(II) with symmetrical, bipodal N′,N′,N‴,N‴-tetraalkyl-N,N″-phenylenedicarbonylbis(thiourea). J. Chem. Soc. Dalton Trans. 1999, 3157–3161. [Google Scholar] [CrossRef]
- Rodenstein, A.; Griebel, J.; Richter, R.; Kirmse, R. Synthese, Struktur und EPR-Untersuchungen von binuklearen Bis(N,N,N‴,N‴-tetraisobutyl-N′,N″-isophthaloylbis(thioureato))-Komplexen des CuII, NiII, ZnII, CdII und PdII. Z. Anorg. Allg. Chem. 2008, 634, 867–874. [Google Scholar] [CrossRef]
- Koch, R.K.; Hallale, O.; Bourne, S.A.; Miller, J.; Bacsa, J. Self-assembly of 2:2 metallomacrocyclic complexes of NiII and PdII with 3,3,3′,3′-tetraalkyl-1,1′-isophthaloylbis(thioureas). Crystal and molecular structures of cis-[Pd(L2-S,O)]2 and the adducts of the corresponding NiII complexes: [Ni(L1-S,O)(pyridine)2]2 and [Ni(L1-S,O)(4-dimethylaminopyridine)2]2. J. Mol. Struct. 2001, 561, 185–196. [Google Scholar]
- Westra, A.N.; Bourne, S.A.; Koch, K.R. First metallamacrocyclic complexes of Pt(iv) with 3,3,3′,3′-tetraalkyl-1,1′-phenylenedicarbonylbis(thioureas): Synthesis by direct or electrolytic oxidative addition of I2, Br2 and Cl2. Dalton Trans. 2005, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Keskin, E.; Solmaz, U.; Gumus, I.; Arslan, H. Di- and tetra-nuclear oxorhenium(V) complexes of benzoylthiourea derivative ligands: Synthesis, structural characterization, and catalytic applications. Polyhedron 2022, 219, 115786. [Google Scholar] [CrossRef]
- Teixeira, E.I.; Schwalm, C.S.; Casagrande, G.A.; Tirloni, B.; Schwade, V.D. Binuclear isophthaloylbis(N,N-diphenylthioureate) transition metal complexes: Synthesis, spectroscopic, thermal and structural characterization. J. Mol. Struct. 2020, 1210, 127999. [Google Scholar] [CrossRef]
- Pham, C.T.; Roca Jungfer, M.; Abram, U. Indium(III) {2}-Metallacryptates Assembled from 2,6-Dipicolinoyl-bis(N,N-diethylthiourea. New J. Chem. 2020, 44, 3672–3680. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, T.H.; Trieu, T.N.; Matsumoto, K.; Nguyen, H.H. Syntheses, Structures, and Magnetism of Trinuclear Zn2Ln Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea). Z. Anorg. Allg. Chem. 2019, 645, 1072–1078. [Google Scholar] [CrossRef]
- Jesudas, J.J.; Pham, C.T.; Hagenbach, A.; Abram, U.; Nguyen, H.H. Trinuclear ‘CoIILnIIICoII’ Complexes (Ln = La, Ce, Nd, Sm, Gd, Dy, Er and Yb) with 2,6-Dipicolinoyl-bis(N,N-diethylthiourea)—Synthesis, Structures and Magnetism. Inorg. Chem. 2020, 58, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.T.; Nguyen, T.H.; Matsumoto, K.; Nguyen, H.H. CuI/CuII Complexes with Dipicolinoylbis(N,N-diethylthiourea): Structures, Magnetism, and Guest Ion Exchange. Eur. J. Inorg. Chem. 2019, 2019, 4142–4146. [Google Scholar] [CrossRef]
- Le, C.D.; Pham, C.T.; Nuyen, H.H. Zinc(II) {2}-Metallacoronates and {2}-Metallacryptates based on Dipicolinoylbis(N,N-diethylthiourea): Structures and Biological Activities. Polyhedron 2019, 173, 114143. [Google Scholar] [CrossRef]
- Sucena, S.F.; Demirer, T.I.; Baitullina, A.; Hagenbach, A.; Grewe, J.; Spreckelmeyer, S.; März, J.; Barkleit, A.; daSilva Maia, P.I.; Nguyen, H.H.; et al. Gold-based Coronands as Hosts for M3+ Metal Ions: Ring Size Matters. Molecules 2023, 28, 5421. [Google Scholar] [CrossRef] [PubMed]
- Noufele, C.N.; Hagenbach, A.; Abram, U. Uranyl Complexes with Aroylbis(N,N-dialkylthioureas). Inorg. Chem. 2018, 57, 12255–12269. [Google Scholar] [CrossRef] [PubMed]
- Noufele, C.N.; Schulze, D.; Roca Jungfer, M.; Hagenbach, A.; Abram, U. Bimetallic Uranium Complexes with 2,6-Dipicolinoylbis(N,N-dialkylthioureas). Molecules 2024, 29, 5001. [Google Scholar] [CrossRef]
- Noufele, C.N.; Roca Jungfer, M.; Nguyen, H.H.; Hagenbach, A.; Abram, U. Uranium-mediated Thiourea/Urea Conversion on Chelating Ligands. Inorganics 2024, 12, 295. [Google Scholar] [CrossRef]
- Degretto, S.; Baracco, L.; Graziani, R.; Celon, E. Preparation and Characterization of some Pyridine-2,3-dicarboxylato Thorium(IV) Complexes. Transition Met. Chem. 1978, 3, 351–354. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Alvarez, S. Stereochemistry of Compounds with Coordination Number Ten. Chem. Eur. J. 2009, 15, 7470–7480. [Google Scholar] [CrossRef] [PubMed]
- Zabrodsky, H.; Peleg, S.; Avnir, D. Continuous Symmetry Measures. J. Am. Chem. Soc. 1992, 114, 7843–7851. [Google Scholar] [CrossRef]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S. Polyhedra in (inorganic) chemistry. Dalton Trans. 2005, 2209–2233. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. Shape—Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools, Version 2.1. University of Barcelona. Available online: https://www.ee.ub.edu/downloads/ (accessed on 10 December 2024).
- Noufele, C.N.; Pham, C.T.; Abram, U. Bis{µ-(2,2′-bipyridine-1κ2N,N‘)-(6,6′-dicarbonyl-1κ2O,O‘:2κO‘)bis(N,N-diethylthioureato-2κS)}(acetato-1κO)(µ-acetato-1κO:2-κO‘)(methanol-2κO)thoriumnickel. Molbank 2025, 2025, M1948. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- APEX 3 Software Suite, version 1.0; Bruker: Billerica, MA, USA, 2016.
- Coppens, P. The Evaluation of Absorption and Extinction in Single-Crystal Structure Analysis. Crystallographic Computing; Muksgaard: Copenhagen, Denmark, 1979. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]


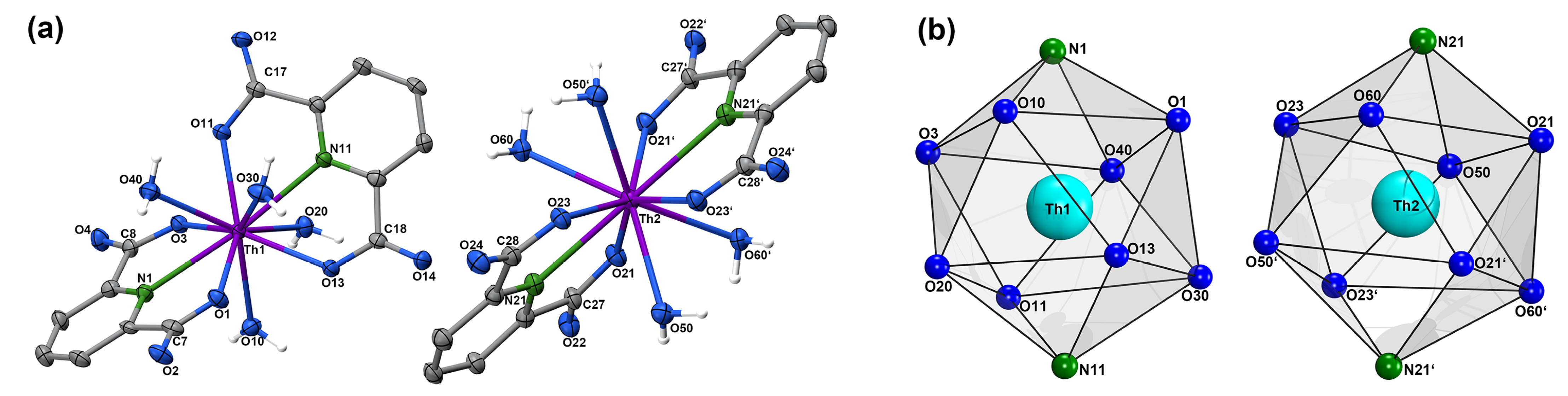
| Th1–O1 | 2.427(4) | Th1–N1 | 2.629(5) | Th1–O3 | 2.432(4) | Th1–O11 | 2.411(4) | Th1–N11 | 2.620(5) |
| Th1–O13 | 2.437(4) | Th1–O10 | 2.551(4) | Th1–O20 | 2.583(5) | Th1–O30 | 2.581(4) | Th1–O40 | 2.503(5) |
| O1–C7 | 1.270(7) | C7–O2 | 1.242(7) | O3–C8 | 1.295(7) | C8–O4 | 1.236(7) | O11–C17 | 1.290(7) |
| C17–O12 | 1.228(6) | O13–C18 | 1.297(7) | C18–O14 | 1.234(7) | Th2–O21 | 2.419(4) | Th2–N21 | 2.629(5) |
| Th2–O23 | 2.417(4) | Th2–O50 | 2.544(4) | Th2–O60 | 2.583(4) | O21–C27 | 1.288(7) | C27–O22 | 1.234(7) |
| O23–C28 | 1.277(7) | C28–O24 | 1.238(7) |
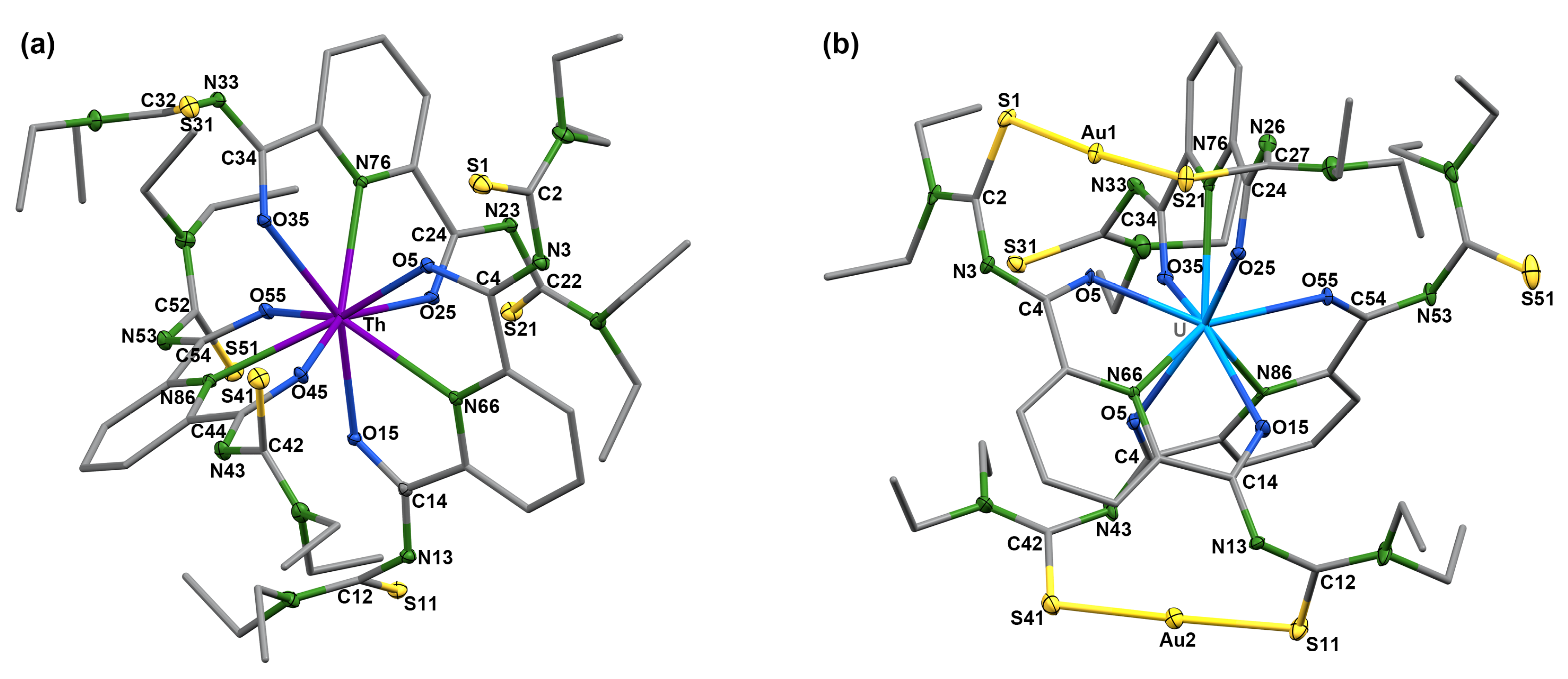
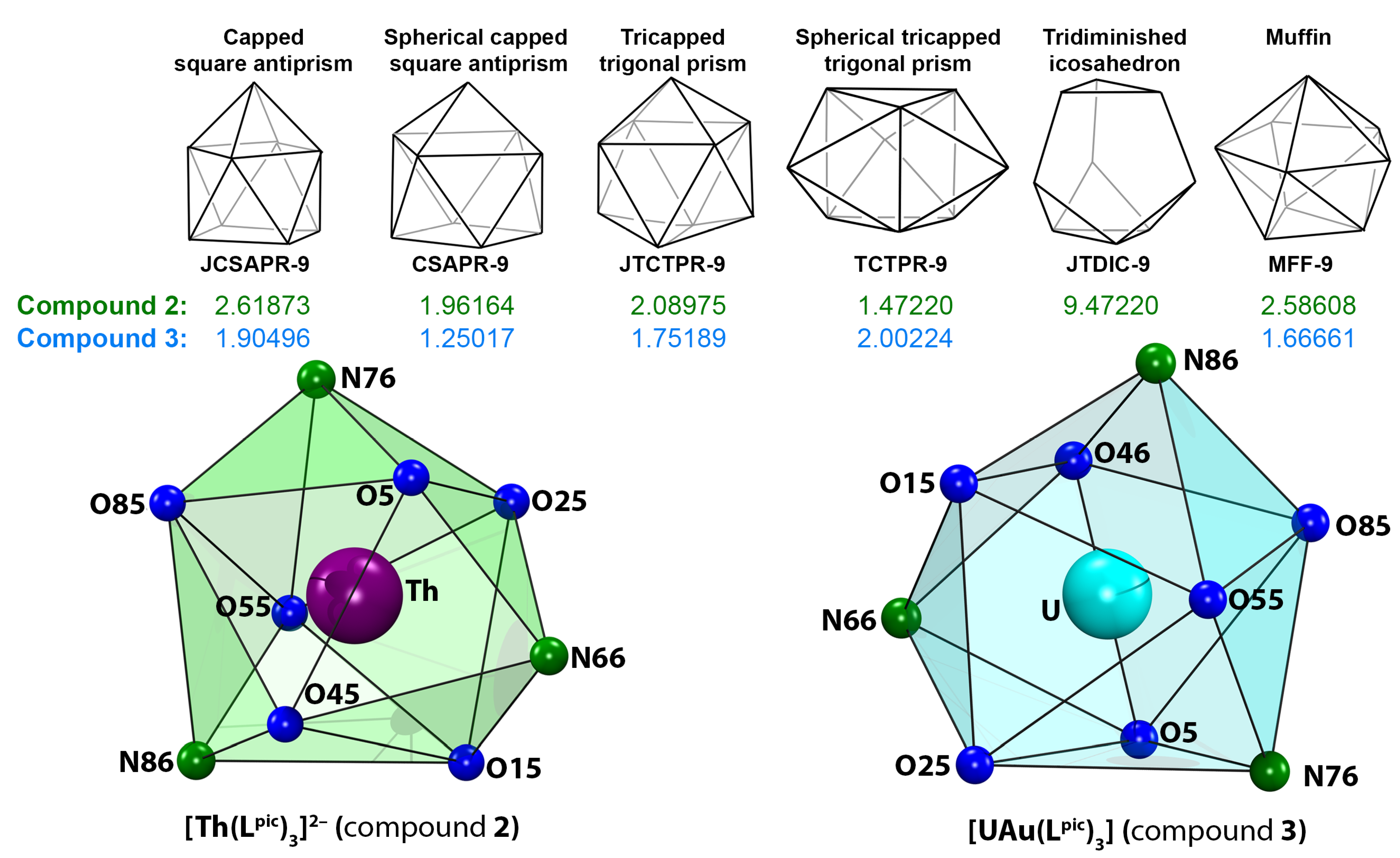
| Compound 2 | |||||||||
| Th–O5 | 2.426(1) | Th–O15 | 2.4266(9) | Th–N66 | 2.616(1) | Th–O25 | 2.4026(9) | Th1–O35 | 2.4321(9) |
| Th–N76 | 2.596(1) | Th–O45 | 2.4069(9) | Th–O55 | 2.4182(10) | Th–N86 | 2.598(1) | O5–C4 | 1.298(2) |
| C4–N3 | 1.293(2) | N3–C2 | 1.391(2) | C2–S1 | 1.691(2) | O15–C14 | 1.303(2) | C14–N13 | 1.293(2) |
| N13–C12 | 1.390(2) | C12–S11 | 1.706(2) | O25–C24 | 1.299(2) | C24–N23 | 1.290(2) | N23–C22 | 1.389(2) |
| C22–S21 | 1.693(1) | O35–C34 | 1.300(2) | C34–N33 | 1.289(2) | N33–C32 | 1.386(2) | C32–S31 | 1.693(1) |
| O45–C44 | 1.299(2) | C44–N43 | 1.286(2) | N43–C42 | 1.286(2) | C42–S41 | 1.7080(2) | O55–C54 | 1.298(2) |
| C54–N53 | 1.292(2) | N53–C52 | 1.392(2) | C52–S51 | 1.711(2) | ||||
| Compound 3 | |||||||||
| U–O5 | 2.336(5) | U–O15 | 2.381(5) | U–N66 | 2.576(6) | U–O25 | 2.414(5) | U–O35 | 2.348(5) |
| U–N76 | 2.542(6) | U–O45 | 2.374(5) | U–O55 | 2.333(5) | U–N86 | 2.551(6) | O5–C4 | 1.286(9) |
| C4–N3 | 1.30(1) | N3–C2 | 1.35(1) | C2–S1 | 1.729(9) | O15–C14 | 1.282(9) | C14–N13 | 1.29(1) |
| N13–C12 | 1.37(1) | C12–S11 | 1.734(9) | O25–C24 | 1.285(9) | C24–N23 | 1.30(1) | N23–C22 | 1.35(1) |
| C22–S21 | 1.731(8) | O35–C34 | 1.294(9) | C34–N33 | 1.29(1) | N33–C32 | 1.39(1) | C32–S31 | 1.676(9) |
| O45–C44 | 1.295(9) | C44–N43 | 1.30(1) | N43–C42 | 1.37(1) | C42–S41 | 1.736(9) | O55–C54 | 1.294(9) |
| C54–N53 | 1.29(1) | N53–C52 | 1.29(1) | C52–S51 | 1.67(1) | Au1–S1 | 2.285(2) | Au1–S21 | 2.293(2) |
| Au2–S11 | 2.283(2) | Au2–S41 | 2.281(2) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noufele, C.N.; März, J.; Abram, U. Thorium(IV) and Uranium(IV) Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea) Ligands. Molbank 2025, 2025, M1957. https://doi.org/10.3390/M1957
Noufele CN, März J, Abram U. Thorium(IV) and Uranium(IV) Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea) Ligands. Molbank. 2025; 2025(1):M1957. https://doi.org/10.3390/M1957
Chicago/Turabian StyleNoufele, Christelle Njiki, Juliane März, and Ulrich Abram. 2025. "Thorium(IV) and Uranium(IV) Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea) Ligands" Molbank 2025, no. 1: M1957. https://doi.org/10.3390/M1957
APA StyleNoufele, C. N., März, J., & Abram, U. (2025). Thorium(IV) and Uranium(IV) Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea) Ligands. Molbank, 2025(1), M1957. https://doi.org/10.3390/M1957






