Abstract
The target compound, N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine, was synthesized in two steps from N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine by a nucleophilic aromatic substitution, yielding N-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine which was characterized by 1H NMR, 13C NMR, 19F NMR, and HRMS. Subsequent reduction yielded the target molecule, N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine. The structure of the target compound was confirmed by 1H NMR, 13C NMR, 19F NMR, HSQC, HMBC, HRMS, and FTIR.
1. Introduction
The quinazoline scaffold is found in many biologically active molecules, such as compounds that have bronchodilatory activity [1,2], antimycobacterial activity [3], or antihypertensive activity [4]. Furthermore, quinazoline derivatives are attractive anticancer agents due to their ability to act as protein kinase inhibitors [5,6]. For example, the N-(3-chloro-4-fluorophenyl)quinazolin-4-amine derivatives gefitinib [7,8,9], afatinib [10,11,12], and dacomitinib [12,13,14] are FDA-approved drugs used in the treatment of non-small cell lung cancer (Figure 1).
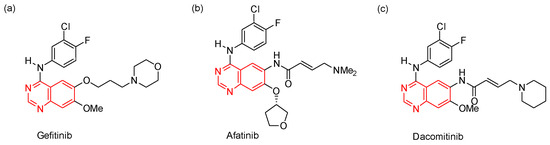
Figure 1.
Examples of FDA-approved quinazoline-based anticancer drugs.
While sulfur-substituted quinazolines have also been investigated as inhibitors of tyrosine kinases or epidermal growth factor receptors, their oxygen analogs have proven to be superior anticancer drug candidates [15,16,17]. We were, however, more interested in potential antibacterial or antimicrobial drug candidates for which sulfur-substituted quinazolines have shown some promise [18].
2. Results and Discussion
The target compound N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) was prepared as an extension of the previous work in our group [19,20] to include biologically relevant scaffolds such as quinazolines. As illustrated in Scheme 1, commercially available N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine (1) was treated with (4-methoxyphenyl)methanethiol which, upon the addition of aqueous sodium hydroxide, selectively afforded N-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine (2) by a nucleophilic aromatic substitution reaction. Compound 2 was isolated in 99% yield as a bright yellow powder and fully characterized by 1H NMR (Supplementary Information, Figure S1),13C NMR (Supplementary Information, Figure S2), 19F NMR (Supplementary Information, Figure S3), and HRMS (Supplementary Information, Figure S4). The subsequent reduction of 2 in the presence of iron powder and ammonium chloride yielded the target compound N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) quantitatively in form of a pale yellow powder. Compounds 2 and 3 are stable in air at room temperature for more than a year (as determined by 1H NMR). Molecules 2 and 3 are also stable in the presence of water such as humid air at room temperature for over a year (as determined by 1H NMR) and in boiling water for over 24 h (as determined by 1H NMR).
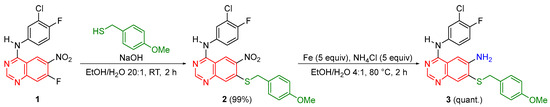
Scheme 1.
Synthesis of N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3).
The molecular structure of the target compound N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) was in agreement with its 1H NMR (Figure 2 and Figure S5). The NH proton at 9.54 ppm and the NH2 protons at 5.47 ppm were confirmed by the absence of a cross peak for these protons in the HSQC spectrum (Supplementary Information, Figure S9). Additionally, upon heating a sample of 3 in D2O at 100 °C for 24 h, partial H–D exchange (approximately 33%) is observed for these NH and NH2 protons. All quinazoline protons appear as singlets in the 1H NMR spectrum while the protons of the N-aryl group are coupled to the protons and the fluorine atom on the same ring. The methylene and methyl protons of the p-methoxylbenzyl group are visible as singlets at 4.30 and 3.72 ppm, respectively. Meanwhile, the aromatic protons of the p-methoxylbenzyl group are easily identified as the two doublets integrating to two protons each at 7.33 and 6.88 ppm, respectively.
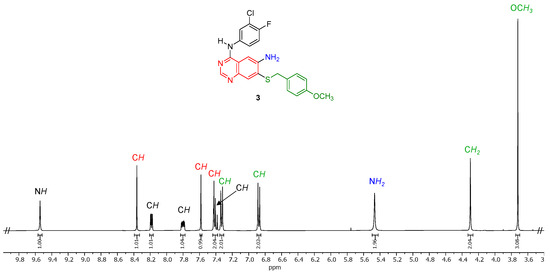
Figure 2.
Expanded 1H NMR spectrum of N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) (400 MHz, 298 K, DMSO-d6).
The 13C NMR spectrum (Figure 3 and Figure S6) of N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) was also in agreement with the molecular structure. Assignment of the individual carbon signals to the various aromatic components of 3 was aided by HSQC (Supplementary Information, Figure S9) and HMBC (Supplementary Information, Figure S10). Notable features include the five doublets for the fluorine-containing aromatic ring, of which the doublet at 152.9 ppm with a coupling constant of 242 Hz can be clearly identified as the carbon directly bound to fluorine. The p-methoxybenzyl group is visible at first glance by the presence of the methoxy carbon at 55.1 ppm and the methylene carbon at 35.5 ppm, as well as the aromatic carbons at 130.2 and 113.9 ppm, respectively.
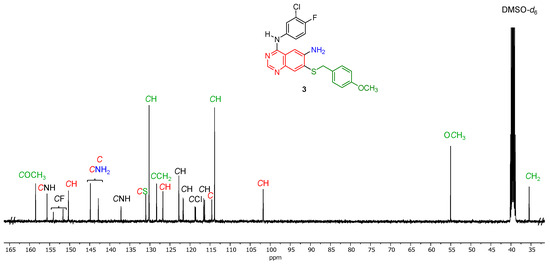
Figure 3.
Expanded 13C{1H} NMR spectrum of N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) (100 MHz, 298 K, DMSO-d6).
As expected, the 19F NMR spectrum (Supplementary Information, Figure S7) shows one aromatic C-F peak (at −123.91 ppm). Additionally, HRMS (Supplementary Information, Figure S10) confirmed the expected atomic composition of 3. The IR spectrum (Supplementary Information, Figure S11) confirms the presence of the NH (medium absorption) which overlaps with the NH2 (weak absorption).
3. Materials and Methods
3.1. General Information and Analyses
Reagents and solvents were purchased from Fisher Scientific or TCI Chemicals and used as supplied. Ethanol for the nucleophilic aromatic solution was used as supplied and not degassed prior to use.
Melting points were obtained using a MEL-TEMP apparatus (Cambridge, MA, USA) and are uncorrected. The 1H NMR, 13C{1H} NMR, and 19F{1H} NMR spectra were all recorded on a 400 MHz Bruker Avance III spectrometer (Bruker, Billerica, MA, USA) with a 5 mm liquid-state Smart Probe at 298 K. Chemical shifts (δH, δC) are expressed in parts per million (ppm) and reported in the 1H NMR spectra relative to the resonance of the residual protons of the DMSO-d6 (δH = 2.50 ppm) or in the 13C{1H} NMR spectra relative to the resonance of the deuterated solvent DMSO-d6 (δC = 39.52 ppm). Chemical shifts in the 19F{1H} NMR spectra are reported relative to the internal standard fluorobenzene (δF = −113.15). High-Resolution Mass Spectrometry (HRMS) data were obtained on an LTQ Orbitrap XL (Thermo Fisher Scientific, Waltham, MA, USA) in FT Orbitrap Mode at a resolution of 100′000. IR spectra were measured in solid form on a Cary 630 FTIR (Agilent, Santa Clara, CA, USA).
3.2. Synthesis of N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine (2)
A 500 mL round-bottom flask equipped with a stir bar was loaded with 8.417 g of N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine (25.00 mmol, 1 equiv) and 200 mL of ethanol and placed under an atmosphere of argon. A total of 4.241 g of (4-methoxyphenyl)methanethiol (27.5 mmol, 1.1 equiv) was added with a syringe, followed by a dropwise addition of a solution of 1.100 g of NaOH (27.5 mmol, 1.1 equiv) in 10 mL of H2O. The reaction mixture was stirred at room temperature for 2 h, after which TLC indicated the completion of the reaction. The solid was filtered off, washed with H2O, followed by ethanol and, finally, diethyl ether to yield the product in 99% yield (11.604 g, 24.64 mmol) in the form of a bright yellow powder, m.p. 210–211 °C.
1H NMR (400 MHz, DMSO-d6, 298 K): δ = 10.39 (s, 1H), 9.51 (s, 1H), 8.67 (s, 1H), 8.14 (d, J = 4.8 Hz, 1H), 7.82–7.79 (m, 2H), 7.45 (t, J = 9.1 Hz, 1H), 7.40 (d, J = 8.6 Hz, 2H), 6.92 (d, J = 8.6 Hz, 2H), 4.39 (s, 2H), 3.74 (s, 3H); 13C{1H} NMR (100 MHz, DMSO-d6, 298 K): δ = 158.8, 157.9, 157.8, 153.6 (d, J = 244.1 Hz), 151.4, 142.3, 140.7, 135.8, 130.5, 126.4, 124.5, 123.7, 123.0, 122.4 (d, J = 6.9 Hz), 118.9 (d, J = 18.4 Hz), 116.5 (d, J = 21.7 Hz), 114.1, 110.8, 55.1, 35.9; 19F{1H} NMR (376 MHz, DMSO-d6, 298 K, referenced to C6H5F): δ = −121.73. HRMS (ESI) m/z calculated for [M + H]+ = [C22H17ClFN4O3S]+ 471.0687; observed, 471.0687 (0 ppm).
3.3. Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3)
A 500 mL round-bottom flask equipped with a stir bar was loaded with 11.106 g of N-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine (23.58 mmol, 1 equiv), 6.308 g of NH4Cl (117.9 mmol, 5.0 equiv), and 250 mL of EtOH/H2O (4:1). The reaction flask was placed into an oil bath set to 80 °C, and 6.595 g of iron power (117.92 mmol, 5.0 equiv) was added while stirring. Then, the reaction flask was fitted with a reflux condenser and the reaction was stirred under argon at 80 °C until TLC indicated a complete reduction after 2 h of reaction time. After cooling to room temperature, the reaction mixture was filtered through celite and concentrated under reduced pressure. The residue was then basified with 1M NaOH and extracted twice with dichloromethane, dried over MgSO4, and evaporated. After washing with hexanes, the product was obtained quantitatively in the form of a pale-yellow powder, m.p. 187 °C.
1H NMR (400 MHz, DMSO-d6, 298 K): δ = 9.54 (s, 1H), 8.36 (s, 1H), 8.19 (dd, J = 6.9, 2.6 Hz, 1H), 7.82–7.78 (m, 1H), 7.58 (s, 1H), 7.44–7.38 (m, 2H), 7.33 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.7 Hz, 2H), 5.47 (s, 2H), 4.30 (s, 2H), 3.72 (s, 3H); 13C{1H} NMR (100 MHz, DMSO-d6, 298 K): δ = 158.5, 155.6, 152.9 (d, J = 242.1 Hz), 150.3, 144.9, 142.9, 137.2 (d, J = 3.0 Hz), 131.0, 130.2, 128.3, 126.8, 122.8, 121.7 (d, J = 6.7 Hz), 118.7 (d, J = 18.1 Hz), 116.5 (d, J = 21.6 Hz), 114.6, 113.9, 101.8, 55.1, 35.5; 19F{1H} NMR (376 MHz, DMSO-d6, 298 K, referenced to C6H5F): δ = −123.91. HRMS (ESI) m/z calculated for [M + H]+ = [C22H19ClFN4OS]+ 441.0947; observed, 441.0948 (0.2 ppm).
4. Conclusions
In summary, we have prepared the target compound N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) in two steps from N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine (1) by a high-yielding nucleophilic aromatic substitution with a subsequent reduction. Both the intermediate N-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine (2) and the target molecule N4-(3-chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3) were characterized by 1H NMR, 13C NMR, 19F NMR, and HRMS. Additionally, the HSQC and HMBC of compound 3 allowed for the assignment of the 1H and 13C signals, while FTIR showed the presence of the N-H bonds. Future work could include studying the antibacterial properties of compounds 2 and 3.
Supplementary Materials
Figure S1: 1H NMR spectrum of 2; Figure S2: 13C NMR spectrum of 2; Figure S3: 19F NMR spectrum of 2; Figure S4: HRMS of 2; Figure S5: 1H NMR spectrum of 3 (full scale); Figure S6: 13C NMR spectrum of 3 (full scale); Figure S7: 19F NMR spectrum of 3; Figure S8: HSQC (aromatic carbons) of 3; Figure S9: HBBC of 3; Figure S10: HRMS of 3; Figure S11: FTIR of 3.
Author Contributions
Conceptualization, J.L.B.; methodology, J.L.B.; validation, J.L.B.; investigation, J.L.B. and S.T.; resources, J.L.B.; data curation, J.L.B.; writing—original draft preparation, J.L.B.; writing—review and editing, J.L.B. and S.T.; supervision, J.L.B.; project administration, J.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We are grateful to the staff of the NMR facility at Oklahoma State University for maintaining the NMR instrumentation and the staff of the Proteomics/Mass Spectrometry Core Facility for assistance in measuring HRMS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bahekar, R.H.; Rao, A.R.R. Synthesis, Evaluation and Structure-Activity Relationships of 5-Alkyl-2,3-Dihydroimidazo[1,2-c]Quinazoline, 2,3-Dihydroimidazo[1,2-c]Quinazolin-5(6H)-Thiones and Their Oxo-Analogues as New Potential Bronchodilators. Arzneimittelforschung 2001, 51, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Špulák, M.; Pourová, J.; Vopršálová, M.; Mikušek, J.; Kuneš, J.; Vacek, J.; Ghavre, M.; Gathergood, N.; Pour, M. Novel Bronchodilatory Quinazolines and Quinoxalines: Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2014, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Chikhalia, K.H.; Kumari, P. Synthesis and Biological Evaluation of Novel Quinazoline Derivatives Obtained by Suzuki C–C Coupling. Med. Chem. Res. 2014, 23, 2338–2346. [Google Scholar] [CrossRef]
- Al-Salahi, R.; El-Tahir, K.-E.; Alswaidan, I.; Lolak, N.; Hamidaddin, M.; Marzouk, M. Biological Effects of a New Set 1,2,4-Triazolo[1,5-a]Quinazolines on Heart Rate and Blood Pressure. Chem. Cent. J. 2014, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Shagufta; Ahmad, I. An Insight into the Therapeutic Potential of Quinazoline Derivatives as Anticancer Agents. Med. Chem. Commun. 2017, 8, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, H.; Diab, A.; Al Musaimi, O. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer: Current Use and Future Prospects. Int. J. Mol. Sci. 2024, 25, 10008. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, R.; Piccirillo, M.C.; Sandomenico, C.; Carillio, G.; Montanino, A.; Daniele, G.; Giordano, P.; Bryce, J.; De Feo, G.; Di Maio, M.; et al. Gefitinib in Non Small Cell Lung Cancer. BioMed Res. Int. 2011, 2011, 815269. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sadhukhan, S.; Sonawane, A. 20 Years since the Approval of First EGFR-TKI, Gefitinib: Insight and Foresight. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188967. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Kies, M.S. ZD1839 (IressaTM) in Non-Small Cell Lung Cancer. Oncologist 2002, 7, 9–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, X.; Xiang, Y.; Fang, T.; Liu, J.; Lu, K. Afatinib for the Treatment of NSCLC with Uncommon EGFR Mutations: A Narrative Review. Curr. Oncol. 2023, 30, 5337–5349. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V. Next-Generation Covalent Irreversible Kinase Inhibitors in NSCLC: Focus on Afatinib. BioDrugs 2015, 29, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, Z.; Wang, L.; Yao, Y.; Liu, Y.; Hao, X.Z.; Wang, J.; Xing, P.; Li, J. Efficacy of Dacomitinib in Patients with EGFR-Mutated NSCLC and Brain Metastases. Thorac. Cancer 2021, 12, 3407–3415. [Google Scholar] [CrossRef]
- Shirley, M. Dacomitinib: First Global Approval. Drugs 2018, 78, 1947–1953. [Google Scholar] [CrossRef]
- Smaill, J.B.; Rewcastle, G.W.; Bridges, A.J.; Zhou, H.; Showalter, H.D.H.; Fry, D.W.; Nelson, J.M.; Sherwood, V.; Elliott, W.L.; Vincent, P.W.; et al. Tyrosine Kinase Inhibitors. 17. Irreversible Inhibitors of the Epidermal Growth Factor Receptor: 4-(Phenylamino)Quinazoline- and 4-(Phenylamino)Pyrido[3,2-d]Pyrimidine-6-Acrylamides Bearing Additional Solubilizing Functions. J. Med. Chem. 2000, 43, 1380–1397. [Google Scholar] [CrossRef] [PubMed]
- Ojida, A.; Ono, M.; Watari, K.; Shindo, N.; Fuchida, Y. Novel Compound Useful for Egfr Inhibition and Tumor Treatment. WO2018084321A1, 13 February 2020. [Google Scholar]
- Fakhoury, S.A.; Lee, H.T.; Reed, J.E.; Schlosser, K.M.; Sexton, K.E.; Tecle, H.; Winters, R.T. 4-Phenylamino-Quinazolin-6-Yl-Amides. US2005250761A1, 10 November 2005. [Google Scholar]
- Bhambra, A.; Weaver, G. 5,6,8-Trifluoroquinazolines to Treat Parasitic Infection. WO2024157021A1, 2 August 2024. [Google Scholar]
- Ardón-Muñoz, L.G.; Bolliger, J.L. Synthesis of Benzo[4,5]Thiazolo[2,3-c][1,2,4]Triazole Derivatives via C-H Bond Functionalization of Disulfide Intermediates. Molecules 2022, 27, 1464. [Google Scholar] [CrossRef]
- Ardón-Muñoz, L.G.; Bolliger, J.L. Synthesis of Sulfur Heterocycles by C–H Bond Functionalization of Disulfide Intermediates. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 507–512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).