1. Introduction
Peptide antibodies have emerged as a promising tool in immunology, offering a wide range of applications due to their numerous advantages [
1,
2,
3,
4]. Peptide epitopes are usually linked to a carrier protein such as bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH) in order to elicit a strong immune response leading to high-antibody titers [
5,
6]. Our lab has extensively studied and designed a variety of Sequential Oligopeptide Carriers (SOCs) suitable for binding epitopes that could maintain their spatial arrangement, leading to the production of antigens with great specificity and sensitivity [
7,
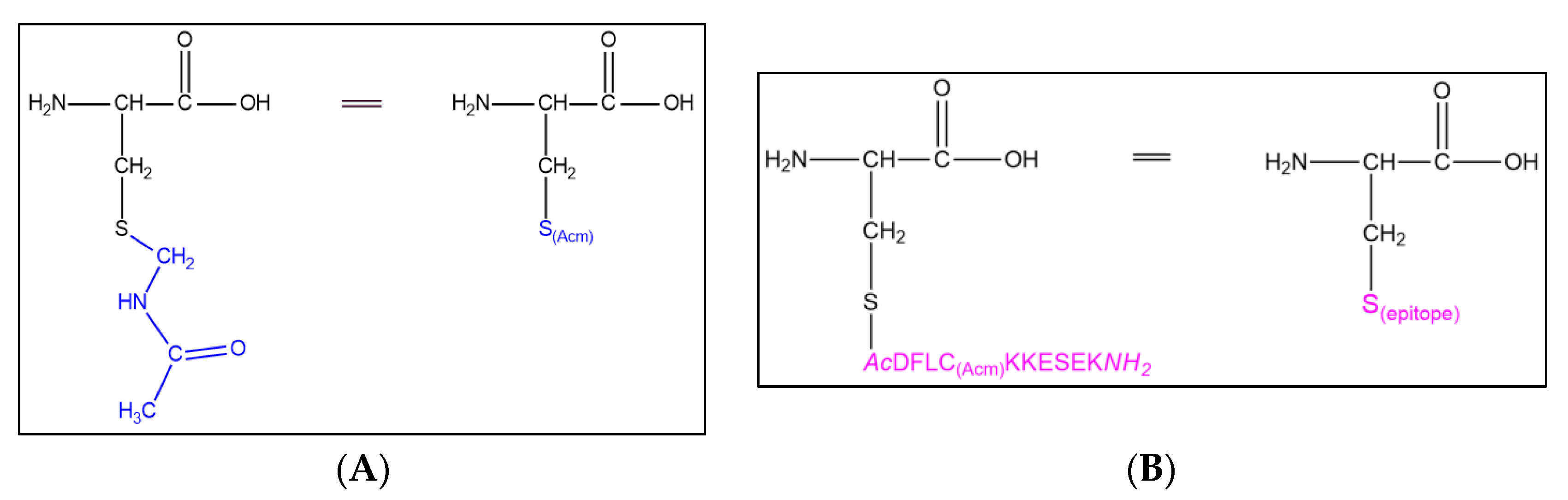
8]. Further research resulted in modifying the initial repetitive moiety Lys-Aib-Gly (Aib: 2-aminoisobutyric acid) to include cysteine residues (CPSOC carrier), allowing for chemoselective reactions such as thioether linkage (
Figure 1) [
9].
In addition, in snake antivenom research, various studies have shown that using purified toxins as antigens leads to antibodies with higher titers and affinity compared to total venom. Building on this concept, several attempts have been made to use peptide epitopes from the most lethal toxins to neutralize their pharmacological activity and serve as an alternative to whole-venom immunization [
10,
11,
12].
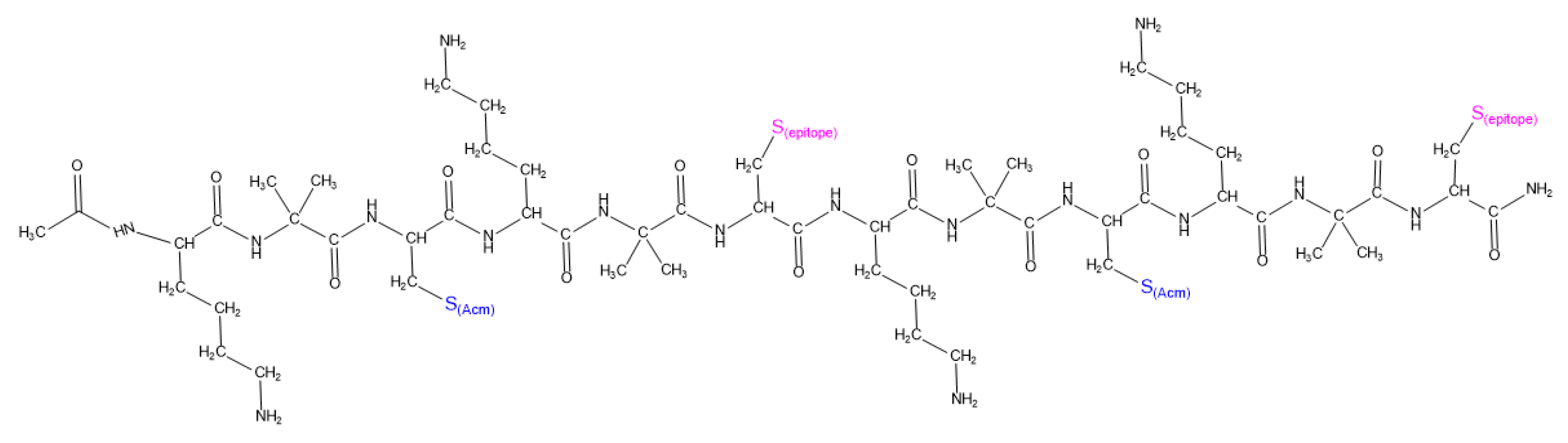
Based on these insights, this study presents the synthesis of [
Ac-K-Aib-C(3,9-
Acm; 6,12-
epitope)]
4-
NH2] (
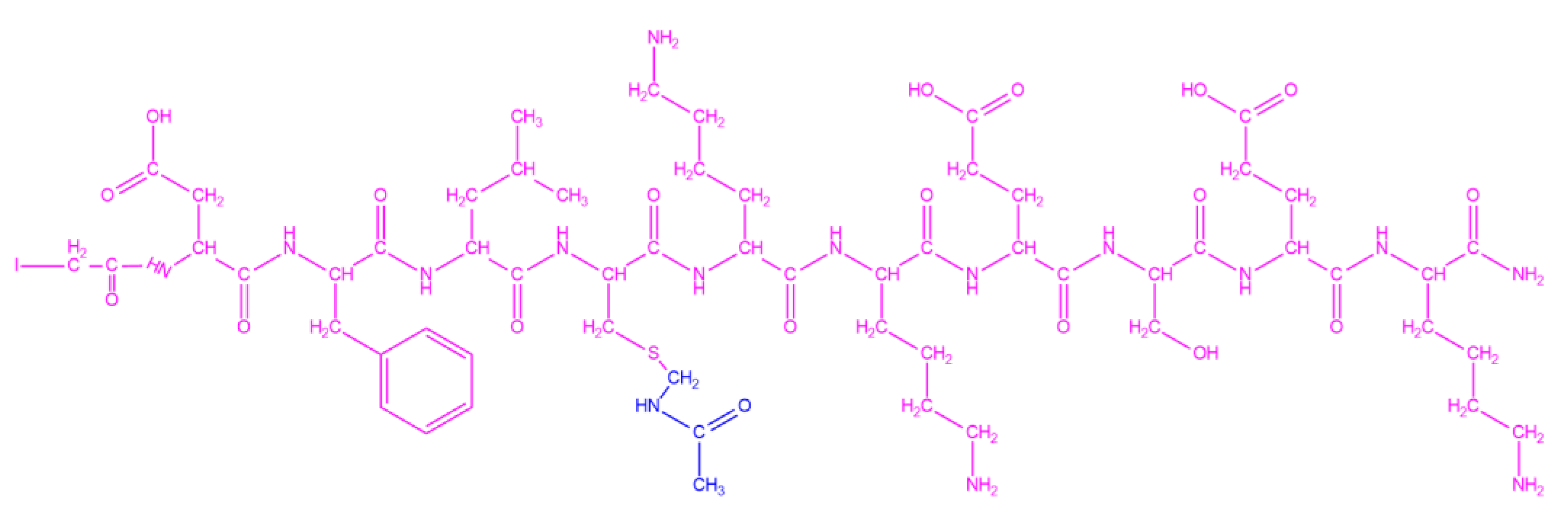
Figure 2) using chemoselective thioether bond formation between the slightly modified CPSOC carrier (CPSOC (3,9 Acm), (Acm: acetamidomethyl) and the iodoacetylated peptide epitope D
128FLCKKESEK
137 (
Figure 3). The latter corresponds to the C-terminal segment of the basic phospholipase A
2, ammodytoxin A, from the V. ammodytes snake species [
13]. The peptide was synthesized according to the SPPS Fmoc/tBu strategy, purified via RP-HPLC, and characterized via HR-MS and NMR spectroscopy. This synthesis represents the initial step towards employing multiple peptide conjugates to induce a robust immune response in hens, leading to the production of IgY antibodies with broad-spectrum antivenom activity.
2. Results and Discussion
2.1. Peptide and Conjugate Synthesis
The desired conjugate was successfully synthesized employing the solid-phase peptide synthesis (SPPS) and chemoselective reaction for thioether bond formation [
9,
14,
15]. The characterization of both peptide and conjugate was accomplished via mass spectrometry (
Supplementary Materials, Figures S1 and S4). HR-ESI-MS, positive (
m/
z): C
58H
94IN
15O
19S (
IAc-D
128FLC
(Acm)KKESEK
137-
NH2): found 488.86; calc. 489.14 for the [C
58H
94IN
15O
19S]
+3; found 732.78; calc. 733.21 for the [C
58H
94IN
15O
19S]
+2; found 1464.57; calc. 1464.43 for the [C
58H
94IN
15O
19S]
+1. HR-ESI-MS, positive (
m/
z): C
176H
297N
49O
53S
6 [
Ac-K-Aib-C(3,9-
Acm; 6,12-
epitope)]
4-
NH2] found 1035.78; calc. 1035.98 for the [C
176H
297N
49O
53S
6]
+4; found 828.81; calc. 828.98 for the [C
176H
297N
49O
53S
6]
+5; found 690.68; calc. 690.98 for the [C
176H
297N
49O
53S
6]
+6. The synthesis yield was 72% for the peptide and 58% for the conjugate.
2.2. 1D and 2D NMR Spectroscopy
To further characterize the synthesized peptide, we conducted NMR spectroscopy. The
1H (δ, ppm) chemical shifts of the peptide epitope
IAc-D
128FLC
(Acm)KKESEK
137-
NH2 can be found in
Table S1 of the
Supplementary Materials. The 1D and 2D NMR spectra are shown in the
Supplementary Materials, Figures S5–S8. Analysis of the NOESY spectrum indicates that the peptide does not adopt a specific conformation as there are no NOE signals between protons of distant amino acids. Additionally, there are no observed interactions between the side chains of amino acids, such as lysine, and protons of distant amino acids.
2.3. Purification
Both the peptide and conjugate were purified via semi-preparative RP-HPLC, and their purity was monitored via analytical RP-HPLC (
Supplementary Materials, Figures S2 and S3). The purification yields were 39% for the peptide and 11% for the conjugate.
3. Materials and Methods
Fmoc amino acid derivatives, Rink amide AM resin and 1-hydroxybenzotriazole (HOBt), were acquired from GL Biochem (Shanghai, China). Trifluoroacetic acid (TFA) and piperidine were purchased from Honeywell, Riedel-de Haen (Seelze, Germany). Dichloromethane (DCM), dimethylformamide (DMF), N,N′-diisopropylcarbodiimide (DIC), and dimethylbenzene (DMB) were purchased from Fluka (Seelze, Germany). Acetonitrile (HPLC grade) and n-hexane were sourced from Labscan (Dublin, Ireland). Acetic acid and iodoacetic acid were obtained from Sigma-Aldrich (Steinheim, Germany), and triisopropylsilane (TIS) was obtained from Acros Organics (Waltham, MA, USA).
3.1. Peptide Synthesis
The peptide synthesis was performed using the step-by-step solid-phase peptide synthesis on the Rink Amide AM resin (substitution 0.58 mmol/g) employing the Fmoc/tBu methodology (Fmoc: 9-fluorenylmethyloxy-carbonyl). N
α-Fmoc-protected amino acids were dissolved in DMF/DCM (1/1,
v/
v) using a molar ratio of Fmoc–amino acid/DIC/HOBt/resin: 3/3/3/1. The Fmoc protecting groups were removed using a solution of 20% (
v/
v) piperidine in DMF. Both the coupling reactions and Fmoc-deprotection steps were monitored via the Kaiser test. To bind the peptide to the carrier, an iodoacetyl group was introduced to the peptide’s N-terminus. For this purpose, iodoacetic acid (ICH
2COOH) was dissolved in DMF/DCM (1/1,
v/
v) and added to the vessel in a ratio of ICH
2COOH/DIC/resin: 10/10/1. The reaction was carried out in the dark for 3 h [
9].
3.2. Peptide Cleavage from the Resin
The peptide cleavage and deprotection of side groups were performed using a solvent mixture of 95% TFA, 2.5% TIS, and 2.5% DMB. The reaction was conducted in the dark at 25 °C. After 3 h, the resin was filtered, and a mixture of DCM/hexane (1:1, v/v) was added to the solution. The mixture was concentrated using a flash evaporator. Afterwards, the peptide was precipitated with cold diethyl ether, dissolved in 2N acetic acid, lyophilized, and stored at 4 °C.
3.3. Thioether Bond Formation
The thioether bond was formed between the previously developed CPSOC carrier [
9], slightly modified (CPSOC (3,9 Acm)), and the antigenic epitope
IAc-DFLC
(Acm)KKESEK-
NH2. Iodoacetyl-peptide (0.0018 mmol/mL) was dissolved in a mixture of H
2O/AcN (1:1,
v/
v), and the pH was adjusted to 8.2 using diisopropylethylamine (DIPEA). The carrier was added gradually, in four portions, at a 20 min interval and in solid form. The reaction was carried out under inert conditions (N
2 atmosphere) for 4 h and was terminated by formic acid until pH reached 2–3.
3.4. High-Performance Liquid Chromatography
The peptide and conjugate were purified via semi-preparative RP-HPLC (Shimadzu, Germany) with a Discovery C18 column (25 cm × 10 mm) and a flow rate of 4.7 mL/min. The chromatograms were acquired at 214 nm, and the gradient elution system used was H2O (0.1% TFA)/acetonitrile (0.1% TFA) from 90/10% to 60/40%. Peptide and conjugate purity were checked via analytical RP-HPLC associated with a C18 Supelco column (25 cm × 3 mm, 5 μm) (Shimadzu, Germany).
3.5. Mass Spectroscopy
A concentration of 2 ppm in H2O (0.1 formic acid) of the peptide and conjugate was injected into the HR-ESI-MS (Thermo Scientific LTQ Orbitrap XL™ system, Thermo Fisher Scientific, Bremen, Germany) to acquire the mass spectra.
3.6. NMR Spectroscopy
NMR experiments were conducted on a Bruker Avance spectrometer at a frequency of 500.13 MHz. Spectra were processed using Topspin 4.2 (Bruker Analytik GmbH, Bremen, Germany). Both one and two dimension experiments (1H, 1H–1H-TOCSY, 1H–1H COSY, 1H–1H NOESY) were acquired at T = 298 K and in a 9:1 (v/v) H2O:D2O solution at a peptide concentration of 5 mM.
4. Conclusions
In conclusion, we successfully developed the branched macromolecule Ac-[K-Aib-C(3,9-Acm; 6,12-epitope)]4-NH2 by conjugating two copies of the peptide epitope IAc-DFLC(Acm)KKESEK-NH2 to the CPSOC (3,9 Acm) carrier. The chemoselective reaction of thioether bond formation was chosen to synthesize the conjugate by linking the peptide epitope, by its modified N-terminus, in two copies to the two free Cys residues of the carrier. Both the peptide epitope and the conjugate were synthesized in satisfactory yields and were of high purity, which underscores the robustness of the synthetic strategy employed.
The present work forms the basis for producing antibodies with broad-spectrum antivenom activity, given that similarly synthesized macromolecules have been successfully used for inducing the immune response with high-titer antibodies and for neutralizing the pharmacological effects of snake toxins [
12].
Supplementary Materials
Figure S1: Mass spectrum of the synthesized peptide IAc-DFLC(Acm)KKESEK-NH2; Figure S2: Analytical chromatogram of the synthesized peptide IAc-DFLC(Acm)KKESEK-NH2; Figure S3: Analytical chromatogram of the conjugate Ac-[K-Aib-C(3,9-Acm; 6,12-epitope)]4-NH2; Figure S4: Mass spectrum of the conjugate Ac-[K-Aib-C(3,9-Acm; 6,12-epitope)]4-NH2; Figure S5: 1H NMR spectrum of the IAc-DFLC(Acm)KKESEK-NH2; Figure S6: 1H 1H COSY NMR spectrum of the IAc-DFLC(Acm)KKESEK-NH2; Figure S7: 1H 1H NOESY NMR spectrum of the IAc-DFLC(Acm)KKESEK-NH2; Figure S8: 1H 1H TOCSY NMR spectrum of the IAc-DFLC(Acm)KKESEK-NH2; Table S1: Chemical shifts of the synthesized peptide IAc-DFLC(Acm)KKESEK-NH2.
Author Contributions
Conceptualization, V.T. and V.M. (Vassilios Moussis); methodology, V.M. (Vasiliki Moulasioti) and E.F.; software, V.M. (Vasiliki Moulasioti) and E.F; validation, V.M. (Vasiliki Moulasioti) and E.F.; formal analysis, V.M. (Vasiliki Moulasioti); investigation, V.M. (Vasiliki Moulasioti); resources, V.T; data curation, V.M. (Vasiliki Moulasioti) and E.F.; writing—original draft preparation, V.M. (Vasiliki Moulasioti); writing—review and editing, V.M. (Vasiliki Moulasioti), E.F., V.M. (Vassilios Moussis) and V.T.; visualization, V.M. (Vasiliki Moulasioti); supervision, V.T.; project administration, V.T., V.M. (Vasiliki Moulasioti) and V.M. (Vassilios Moussis); funding acquisition, V.M. (Vasiliki Moulasioti). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hellenic Foundation for Research and Innovation (HFRI) under the 3rd Call for HFRI PhD Fellowships (Fellowship Number: 5428).
Data Availability Statement
The original contributions presented in the study are included in the article/
Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank The Network of Research Supporting Laboratories, University of Ioannina, for providing access to the NMR and HR-ESI-MS facilities.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hamley, I.W. Peptides for Vaccine Development. ACS Appl. Bio Mater. 2022, 5, 905–944. [Google Scholar] [CrossRef]
- Trier, N.; Hansen, P.; Houen, G. Molecular Sciences Peptides, Antibodies, Peptide Antibodies and More. Int. J. Mol. Sci. 2019, 20, 6289. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Hansen, P.R.; Houen, G. Production and Characterization of Peptide Antibodies. Methods 2012, 56, 136–144. [Google Scholar] [CrossRef]
- Yang, H.; Kim, D.S. Peptide Immunotherapy in Vaccine Development: From Epitope to Adjuvant. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: New York, NY, USA, 2015; Volume 99, pp. 1–14. [Google Scholar]
- Lateef, S.S.; Gupta, S.; Jayathilaka, L.P.; Krishnanchettiar, S.; Huang, J.S.; Lee, B.S. An Improved Protocol for Coupling Synthetic Peptides to Carrier Proteins for Antibody Production Using DMF to Solubilize Peptides. J. Biomol. Tech. JBT 2007, 18, 173. [Google Scholar] [PubMed]
- Adamczyk, O.; Szota, M.; Rakowski, K.; Prochownik, M.; Doveiko, D.; Chen, Y.; Jachimska, B. Bovine Serum Albumin as a Platform for DesigningBiologically Active Nanocarriers—Experimental And Computational Studies. Int. J. Mol. Sci. 2023, 25, 37. [Google Scholar] [CrossRef] [PubMed]
- Sakarellos-Daitsiotis, M.; Tsikaris, V.; Sakarellos, C. A New Circular Helicoid-Type Sequential Oligopeptide Carrier for Assembling Multiple Antigenic Peptides. In Self-Assembling Peptide Systems in Biology, Medicine and Engineering; Aggeli, A., Boden, N., Zhang, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 257–271. ISBN 978-0-306-46890-2. [Google Scholar]
- Alexopoulos, C.; Krikorian, D.; Panou-Pomonis, E.; Sakarellos-Daitsiotis, M.; Sakarellos, C. Innovative, Multifunctional Sequential Oligopeptide Carriers Soc n-I and SOC n-II: Functions-Technology-Perspectives. Protein Pept. Lett. 2005, 12, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Fotou, E.; Moussis, V.; Ntoyhaniari, A.; Zografou, S.; Maltabe, V.; Kouklis, P.; Christoforidis, S.; Tsikaris, V. Intracellular Targets: A Multiple Cargo Transporting Molecule. J. Pept. Sci. 2021, 27, e3359. [Google Scholar] [CrossRef] [PubMed]
- Dias da Silva, W.; De Andrade, S.A.; Megale, Â.A.A.; De Souza, D.A.; Sant’Anna, O.A.; Magnoli, F.C.; Guidolin, F.R.; Godoi, K.S.; Saladini, L.Y.; Spencer, P.J.; et al. Antibodies as Snakebite Antivenoms: Past and Future. Toxins 2022, 14, 606. [Google Scholar] [CrossRef]
- Bermúdez-Méndez, E.; Fuglsang-Madsen, A.; Føns, S.; Lomonte, B.; Gutiérrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef]
- Curin-Serbec, V.; Délot, E.; Faure, G.; Saliou, B.; Gubenšek, F.; Bon, C.; Choumet, V. Antipeptide Antibodies Directed to the C-Terminal Part of Ammodytoxin A React with the PLA2 Subunit of Crotoxin and Neutralize Its Pharmacological Activity. Toxicon 1994, 32, 1337–1348. [Google Scholar] [CrossRef]
- Logonder, U.; Krizaj, I.; Rowan, E.G.; Harris, J.B. Neurotoxicity of Ammodytoxin A in the Envenoming Bites of Vipera Ammodytes Ammodytes. J. Neuropathol. Exp. Neurol. 2008, 67, 1011–1019. [Google Scholar] [CrossRef]
- Hackenberger, C.P.R.; Schwarzer, D. Chemoselective Ligation and Modification Strategies for Peptides and Proteins. Angew. Chem. Int. Ed. 2008, 47, 10030–10074. [Google Scholar] [CrossRef]
- Albericio, F. (Ed.) Solid-Phase Synthesis: A Practical Guide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).