1. Introduction
7-amino, 4-methyl coumarin derivatives have a wide range of applications due to their unique chemical properties [
1,
2,
3]. These compounds have gained significant importance in recent years due to their diverse biological activities. Studies on coumarin derivatives have demonstrated their antitumor [
4], anti-HIV [
5], antibacterial and antifungal [
6,
7], anti-inflammatory [
8,
9], anticoagulant (inhibitors of the enzyme VKOR, vitamin K epoxide reductase) [
10], triglyceride-lowering, and central nervous system stimulant effects. Additionally, hydroxycoumarins have been reported to possess strong antioxidant and protective effects against oxidative stress by scavenging reactive oxygen species [
10].
Chlorine is a very important industrial chemical used in many industries. Its importance extends notably into pharmaceuticals, where it is a key ingredient for drugs that are highly essential in treating a myriad of diseases such as meningitis, cholera, plague, typhoid, bacterial skin infections, and respiratory and nervous system disorders [
11]. Chlorine atoms have been used as metabolically more stable isosteres, replacing hydrogen atoms. Moreover, using chlorine as an isostere for hydrogen can increase potency, and improve solubility [
12].
This would have immense significance for the creation of new hybrid molecules between 7-amino-4-methyl-2H-chromen-2-one and (±)-2-chloro-2-phenylacetyl chloride in the pharmaceutical industry. 7-amino-4-methyl-2H-chromen-2-one is the backbone of numerous bioactive compounds with various pharmacological properties. Its chromenone scaffold has been explored in a drug discovery context because of its versatile nature and potential therapeutic applications. (±)-2-chloro-2-phenylacetyl chloride, on the other hand, introduces a chlorine atom as a bioisostere for hydrogen. This will enhance the metabolic stability and potency of the resulting hybrid molecules. Chlorine, as a bioisostere, often improves the pharmacokinetic properties of compounds by mimicking hydrogen while offering increased resistance to metabolic degradation. Combining these two molecular entities opens up possibilities for the synthesis of novel compounds with enhanced pharmacological profiles, probably leading to the development of more effective drugs for various medical conditions. The synthesis and exploration of hybrid molecules derived from these precursors represent a promising avenue for pharmaceutical research and drug discovery.
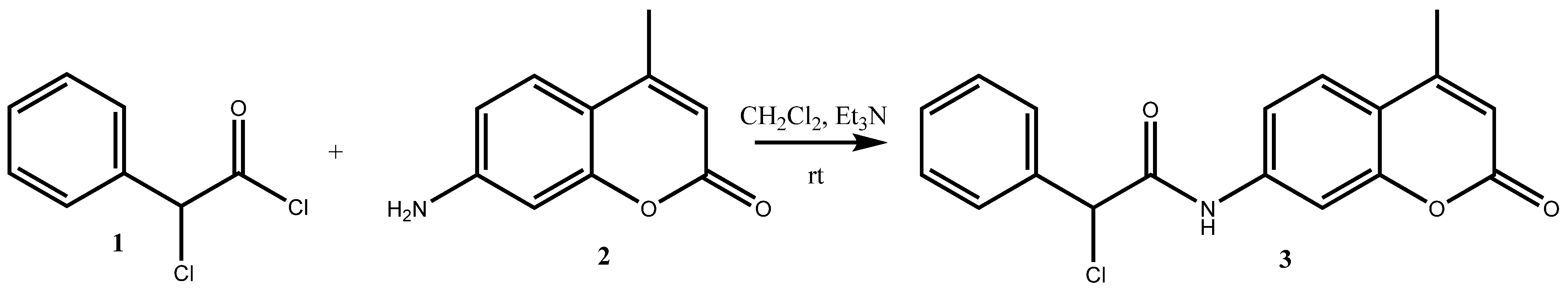
2. Results and Discussion
Herein, we report the successful synthesis of (±)-2-chloro-
N-(4-methyl-2-oxo-2
H-chromen-7-yl)-2-phenylacetamide
3 according to
Scheme 1. The synthesis of this novel hybrid molecule was achieved by a quick and easy-to-perform known method described in the experimental section.
For this purpose, (±)-2-chloro-2-phenylacetyl chloride
1 (1 mmol) is added to a solution of 7-amino-4-methyl-2
H-chromen-2-one
2 in dichloromethane. The reaction mixture was stirred for ten minutes, after which an excess of trimethylamine (1.5 mmol) was cautiously introduced. After 30 min, the TLC analysis confirmed the formation of the final product
3. As we used racemic (±)-2-chloro-2-phenylacetyl chloride, a mixture of two enantiomers was obtained as a product. The literature indicates that α-halo phenylacetic acid chloride does not undergo racemization when exposed to a strong base like diisopropylethylamine [
13].
Upon analyzing the
1H-NMR data of the newly synthesized compound, all 14 hydrogen atoms are distinctly visible (
Figure S1). Furthermore, the
13C-NMR spectrum confirms the structure of the chlorine-containing hybrid molecule
3 (
Figure S2). We also conducted HRMS analysis to verify the mass of compound
3 (
Figure S4). Upon detailed analysis of the MS spectrum of amide molecule
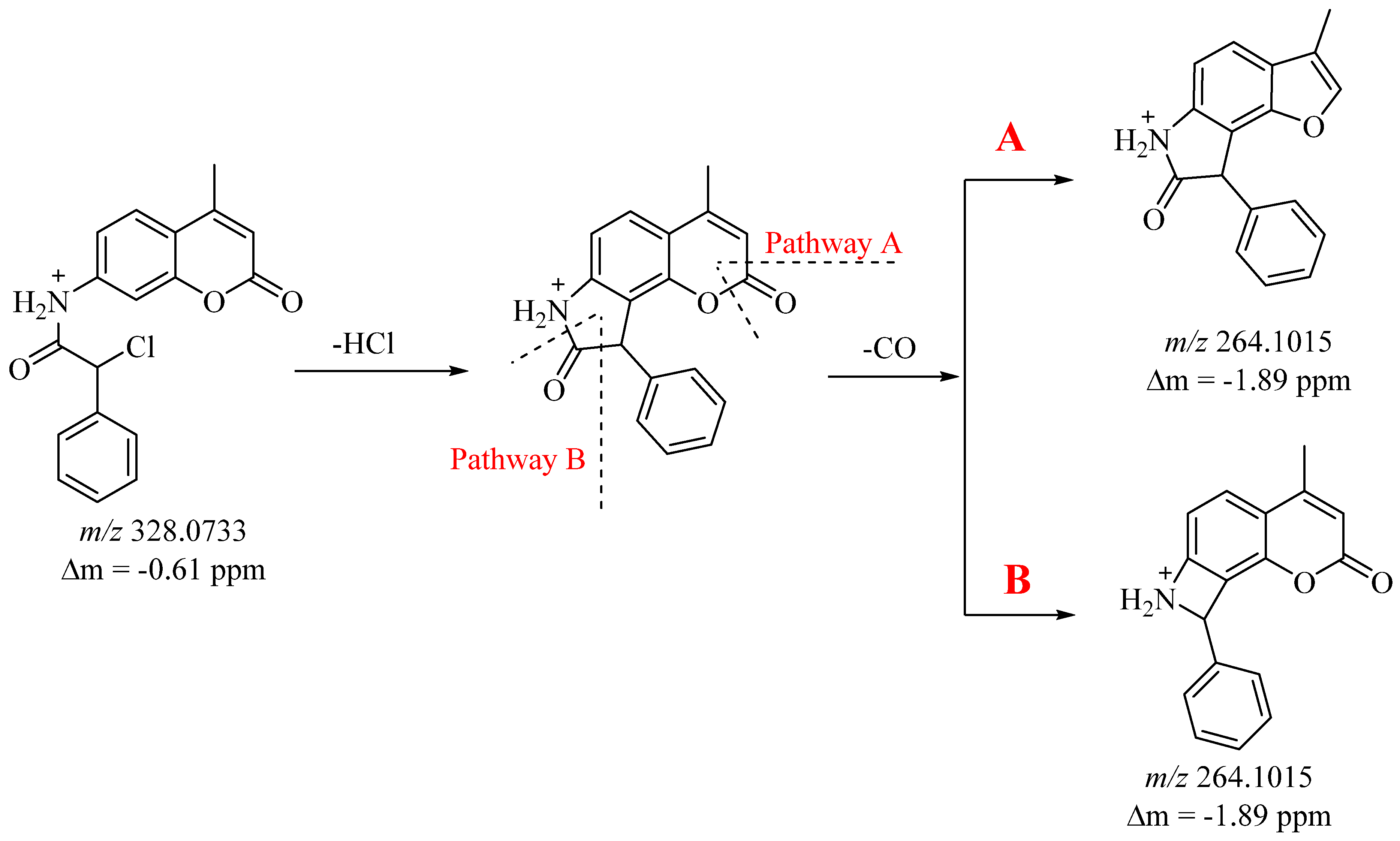
3, three fragmentation mechanisms of the molecular ion are discerned. One involves the cleavage of neutral molecules HCl, CO, and a phenyl fragment from the molecular ion (pathway A and B), while the other two mechanisms entail the cleavage of the NH–C(O)–C(Cl) group, yielding characteristic coumarin and chloro(phenyl)methylium ions (refer to
Figure 1 and
Figure 2). Initially, molecular ion 3 undergoes the loss of neutral molecules HCl and CO, producing the [M+H–HCl–CO]
+ ion with
m/
z 264 (pathway A and B) (refer to
Figure 1 and
Figure 2). The formation of the
m/
z 264 ion is likely attributed to the cleavage of HCl, resulting in the formation of a new five-membered cycle, followed by the subsequent release of a neutral CO molecule. In this scenario, two rearrangement pathways leading to CO release are observed (pathway A and B) (see
Figure 1). In path A, the
m/
z 264 ion releases CO from the coumarin fragment, while in the second case, CO is cleaved from the pyrrolidin-2-one fragment (pathway A and B) (see
Figure 1). Pathway A—the resulting cation
m/
z 264 undergoes sequential loss of neutral CO molecules and the ions
m/
z 236 and
m/
z 208 are generated. The latter loses a phenyl fragment to give cation
m/
z 130. The same ion is produced by different fragmentation pathways. The
m/
z 236 ion initially loses a phenyl fragment and the resulting
m/
z 158 ion further loses a neutral CO molecule and an
m/
z 130 ion is generated. Furthermore, the
m/
z 236 fragment undergoes further fragmentation with the loss of 16 Da (O atom), leading to the ion
m/
z 220. The same ion under ESI-MS conditions suffers loss of a C
6H
6 fragment and a resonance stable cation
m/
z 142 is obtained.
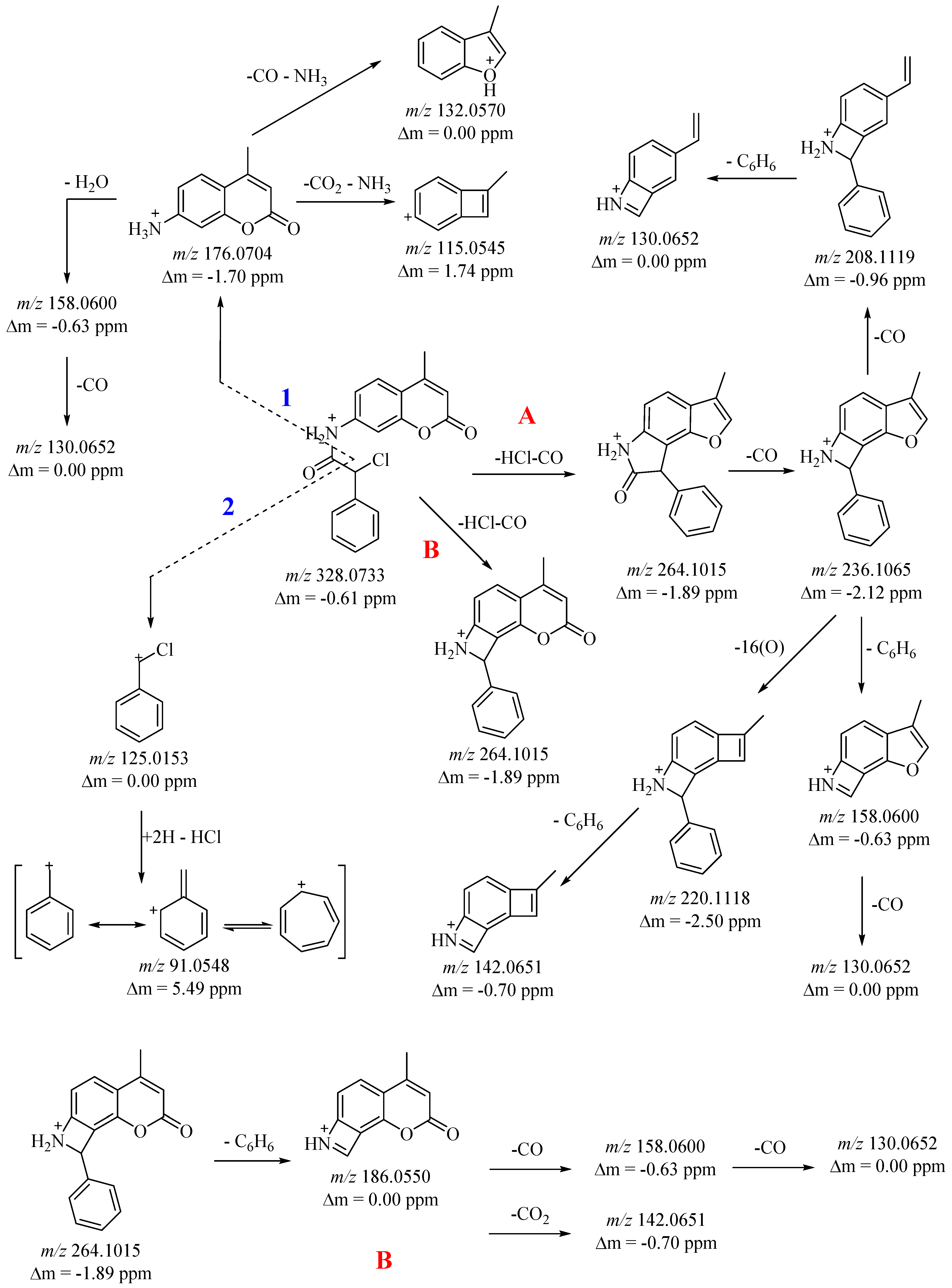
Pathway B is analogous to Pathway A. In this case, the ion
m/
z 264, by cleavage of the sigma bond and loss of the C
6H
6 fragment, results in a stable ion
m/
z 186 (
Figure 2 and
Figure S5). The resulting amide molecule
3 is fragmented by cleavage of the amide bond on the one hand (pathway 1) and on the other by cleavage of the C(O)–C(Cl) sigma bond (pathway 2) (
Figure 2). In doing so, characteristic coumarin fragment cations such as a 4-methyl-2-oxo-2H-chromen-7-aminium cation with
m/
z 176 (pathway 1) and a chloro(phenyl)methylium cation with
m/
z 125 (pathway 2) are generated (
Figure 2 and
Figure S5). Pathway 1—the obtained cation
m/
z 176 in ESI-MS conditions is parallel fragmented undergoing rearrangement with loss of neutral molecules CO, CO
2, NH
3, and H
2O, generating resonance stable cations. Initially, the
m/
z 176 ion loses both CO and NH
3 and leads to the stable 3-methyl-1
H-benzofuran-1-ium (C
9H
8O
•+) radical cation
m/
z 132. Furthermore, the fragment ion
m/
z 176 undergoes further peg grouping with loss of CO
2 and NH
3, generating 7-methylbicyclo [4.2.0]octa-1,3,5,7-tetraen-3-ylium (C
9H
7+) cation
m/
z 115. The ions
m/
z 158 and
m/
z 130 result from sequential loss of H
2O and CO from the fragment ion
m/
z 176 (
Figure 2 and
Figure S5).
Pathway 2—under ESI-MS conditions, a chloro(phenyl)methylium cation accepting two protons cleaves off a neutral HCl molecule generating a phenylmethylium cation which undergoes rearrangement to obtain a stable tropylium cation with
m/
z 91 (
Figure 2 and
Figure S5).
During inflammation there is lysis of lysosomes, which release their constituent enzymes that cause various disorders [
14]. The response of the cells to inflammation will lead to certain pathological manifestations characterized by redness, heat, swelling, and pain with even impaired physiological functions. Numerous disorders, including arthritis, stroke, and cancer, include inflammation as a pathogenic factor. Protein denaturation is closely linked to the initiation of the inflammatory response, which results in a variety of inflammatory disorders, including arthritis [
15]. According to Opie [
16], tissue injury during life might be referable to denaturation of the protein constituents of cells or of intercellular substance. Hence, the ability of a substance to inhibit the denaturation of protein signifies apparent potential for anti-inflammatory activity.
Amide
3 underwent testing for its ability to inhibit albumin denaturation. This method assesses the extent to which albumin can be shielded from denaturation induced by heating. Human albumin was employed for this evaluation. The percentage inhibition of compound
3 is outlined in
Table 1.
The study results are expressed as IC
50 values. Given ibuprofen’s established anti-inflammatory properties, we opted to utilize it as a benchmark for assessing the activity of the new compound
3 (refer to
Table 1). The IC
50 of ibuprofen, estimated as IAD, was 368.66 µM (refer to
Table 1). The findings of our investigation affirm that amide
3 exhibits greater activity compared to ibuprofen (IC
50 208.92 µM) (refer to
Table 1).
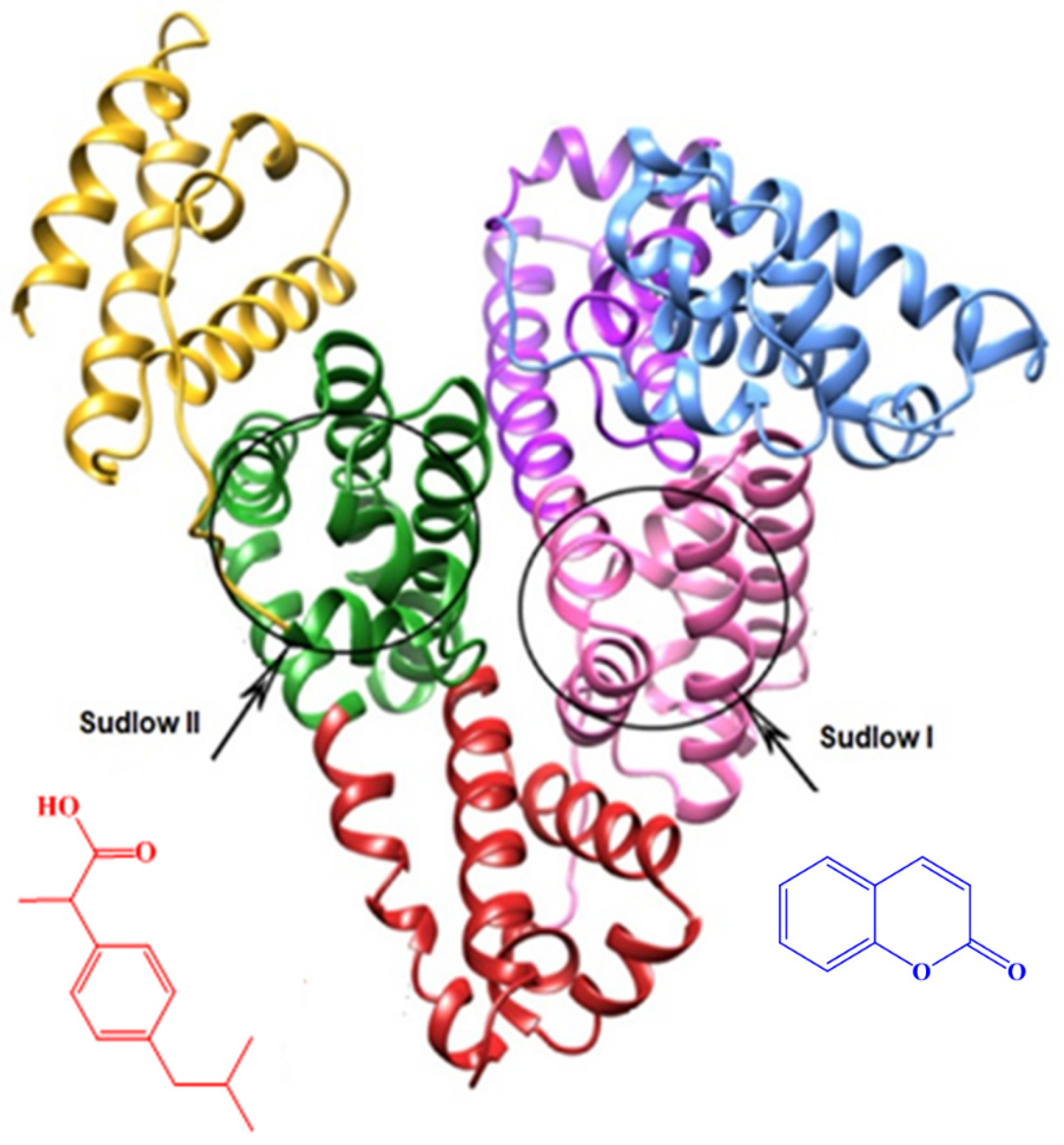
This heightened activity can be attributed to the presence of a coumarin core in its structure, facilitating the allosteric binding of amide
3 to albumin. Evidently, this accounts for the notable stabilization of albumin against denaturation upon heating. Human serum albumin is recognized to possess two primary allosteric binding sites for interacting with external active agents: Sudlow I and II (see
Figure 3). According to the Sudlow nomenclature, Fasano et al. found that bulky heterocyclic molecules bind to Sudlow I (located in subdomain IIA), while aromatic carboxylic acids and phenes bind to Sudlow II (located in subdomain IIIA) (
Figure 3) [
17].
3. Materials and Methods
3.1. Synthesis
All reagents and chemicals were procured from commercial sources (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria) and used without additional purification. NMR spectral data were acquired on a Bruker Avance Neo 400 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA) operating at 400 MHz for 1H NMR and 101 MHz for 13C NMR. The spectra were recorded in DMSO-d6, with chemical shifts referenced in relative ppm to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard, and coupling constants expressed in Hz. NMR measurements were conducted at room temperature (approximately 295 K). Melting point was determined using a Boetius hot stage apparatus and is reported without correction. Absorbance measurements were performed using a Camspec M508 spectrophotometer, Leeds, UK. MS analysis was executed on a Q Exactive Plus high-resolution mass spectrometer (HRMS) with a heated electrospray ionization source (HESI-II) from Thermo Fisher Scientific, Inc., Bremen, Germany, coupled with a Dionex Ultimate 3000RSLC ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). TLC was carried out on 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany).
3.2. In Vitro Biological Assessment
DMSO for analyses and water for HPLC was prepared with a Millipore purifier (Merck Millipore, Burlington, MA, USA). Ibuprofen, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, sodium chloride, potassium chloride, and Tween 80 were purchased from Sigma-Aldrich. Human albumin 20%—BB, 200 g/L was purchased from BB-NCIPD Ltd., Sofia, Bulgaria.
3.3. Synthesis of (±)-R,S-2-chloro-N-(4-methyl-2-oxo-2H-chromen-7-yl)-2-phenylacetamide
A solution of amine 2 (1 mmol, 0.327 g) in dichloromethane (30 mL) was prepared, to which an equivalent amount of (±)-2-chloro-2-phenylacetyl chloride 1 (1 mmol, 0.189 g) was added. After 10 min, triethylamine (1.2 mmol, 0.121 g) was introduced into the solution. Following a 30-min reaction period, the solution was sequentially washed with diluted hydrochloric acid, a saturated solution of Na2CO3, and brine. The combined organic layers were then dried over anhydrous Na2SO4, and the solvent was evaporated under reduced pressure. The resulting new compound was purified by filtration through short-column chromatography using neutral Al2O3.
(±)-R,S-2-chloro-N-(4-methyl-2-oxo-2H-chromen-7-yl)-2-phenylacetamide 3: white solid (m.p. 225–226 °C), yield 90% (0.294 g), 1H NMR (400 MHz, DMSO) δ 10.92 (s, 1H), 7.77−7.66 (m, 2H), 7.65–7.57 (m, 2H), 7.50 (dd, J = 8.7, 2.1 Hz, 1H), 7.47–7.39 (m, 3H), 6.26 (d, J = 1.3 Hz, 1H), 5.80 (s, 1H), 2.38 (d, J = 1.3 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 166.52 (CH(NH)C=O), 160.32 (C=O), 153.98 (Ar), 153.39 (Ar), 141.89 (Ar), 136.78 (Ar), 129.61 (Ar), 129.21 (Ar), 128.55 (Ar), 126.53 (Ar), 116.20 (Ar), 115.97 (Ar), 113.23 (Ar), 106.65 (Ar), 60.45 (CH), 18.41 (CH3). UV λmax, MeOH: 240 (ε = 24,500) nm, 322 (ε = 12,200) nm, 350 (ε = 18,700) nm. HRMS Electrospray ionization (ESI) m/z calculated for [M+H]+ C18H15NO3Cl+ = 328.0735, found 328.0733 (mass error ∆m = −0.61 ppm).
3.4. Inhibition of Albumin Denaturation (IAD)
In vitro analysis of anti-inflammatory activity was assessed as inhibition of albumin denaturation (IAD). The analysis was performed according to the Manolov method [
18] with minor modifications. The experiment was performed with human albumin. The solution of albumin (1%) was prepared in distilled water. Test samples/standards were first dissolved in 1.2 mL of DMSO and then supplemented with 1% Tween 80 in PBS so that the final concentration of the stock solution was 1000 μg/mL. Then, a series of working solutions with different concentrations (20–500 μg/mL) in 1% Tween 80/PBS were prepared. The reaction mixture contained a 2 mL test sample/standard of different concentrations and 1 mL albumin (1%). The mixture was incubated at 37 °C for 15 min and then heated at 70 °C for 15 min in a water bath. After cooling, the turbidity was measured at 660 nm with a spectrophotometer (Camspec M508, UK). The experiment was performed three times. Percentage inhibition of albumin denaturation (IAD) was calculated against control.