Abstract
In the vast field of organic functional materials, viologens are widely recognized as an extremely versatile family of substances, due in part to the possibility of extending conjugation between the terminal pyridinium rings, for instance through the insertion of additional aromatic moieties. In this work, a new, extended viologen with a thiophene core and two acetylene bonds is presented. It was synthesized through a straightforward route, using well-established Sonogashira coupling reactions, and its optical properties were investigated by UV–visible absorption and fluorescence spectroscopy, revealing a very interesting material for diverse fluorescence-related applications.
1. Introduction
Quaternary pyridinium salts are a very well-known and versatile class of substances, with an extraordinary variety of applications in chemistry, biochemistry and material science [1,2], ranging from antibacterial [3] and surfactant agents [4] to catalysts [5] and electrochemical devices [6], among others. In fact, through the functionalization of the pyridyl ring either on the nitrogen or on the carbon atoms, different chemical and electronic properties can be provided, depending on the substituents introduced.
Organic salts possessing at least two quaternarized pyridyl rings, in particular, constitute an especially important subclass of pyridinium compounds, to the point that they have been given the common denomination of viologens, after 1,1′-dimethyl-4,4′-bipyridinium dichloride, also known as methyl viologen, due to the color of its radical cation in solution. The term viologen originally referred to quaternary 4,4′-bipyridinium salts, in which the nitrogen rings are directly connected; however, over time, it has started to be used for related compounds possessing more than two charged pyridyl rings or a conjugated moiety as a spacer between them, since such structures retain the peculiar properties of actual viologens [7]. Among these properties, the most useful one is probably represented by the presence of three reversible and stable redox states, often characterized by different colors, from which another typical behavior of viologens arises, that is, electrochromism [8,9]. This characteristic makes viologens and their analogues a convenient choice for a large number of optoelectronic and energy-related applications, including memory devices [10], molecular switches [11], photocatalysts for hydrogen evolution [12] and different types of batteries [13,14].
As already mentioned, additional conjugated subunits, from simple unsaturated bonds to aromatic rings and macrocycles [15,16,17], can be inserted between the pyridinium rings to obtain the so-called extended viologens, many examples of which have been prepared, characterized and tested in different applications and devices. Therefore, viologens and related systems represent a continuously expanding platform for the construction of tailor-made functional materials.
In the present work, the synthesis and characterization by UV–visible absorption and fluorescence spectroscopy of a thiophene-containing extended viologen featuring triple bonds connecting the three heteroaromatic rings is described. This compound can be easily prepared and shows intense absorption and emission bands, making it a convenient candidate for fluorescence-related applications. In addition, its optical spectrum is compared to that of a similar bipyridinium salt, recently synthesized, characterized by a bithiophene central moiety and interesting light emission [18].
2. Results and Discussion
The synthetic route followed for the target compound is shown in Scheme 1 and includes four straightforward and high-yielding steps, starting from readily affordable 2,5-diiodothiophene. The choice of this compound as a starting material for the construction of an extended, conjugated viologen-type salt, with effective light interaction, was motivated by the widely recognized optoelectronic properties of thiophene derivatives [19], which are particularly exploited as oligomers and polymers for molecular electronics [20], OLEDs [21], emerging photovoltaics [22] and chemo- and biosensors [23]. The heteroaromatic core was linked to the pyridinium rings by palladium-copper-catalyzed Sonogashira cross-coupling [24], thus profiting from both the effectiveness of this reaction and the ability of triple bonds to enable conjugation.
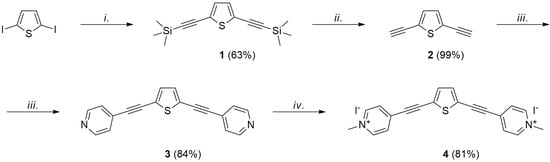
Scheme 1.
Synthesis of compound 4; i. Pd(PPh3)2Cl2 (10 mol%), CuI (10 mol%), TMSA, THF/i-Pr2NH, 60 °C, 48 h; ii. K2CO3, CH3OH, r.t., 16 h; iii. 4-Iodopyridine (2 eq.), Pd(PPh3)2Cl2 (20 mol%), CuI (10 mol%), THF/Et3N, 60 °C, 24 h; iv. CH3I, CH2Cl2, 40 °C, 24 h.
The first step was therefore represented by Sonogashira coupling between 2,5-diiodothiophene and trimethylsilylacetylene, in a tetrahydrofuran/diisopropylamine mixture, using Pd(PPh3)2Cl2 and CuI as catalysts, which afforded the trimethylsilyl-protected diyne 1 as a pale-yellow solid in a satisfactory yield, after column chromatography (63%). The subsequent deprotection in basic conditions, by a reaction with K2CO3 in methanol, followed by chromatographic purification, furnished the corresponding terminal diyne 2, in nearly quantitative yield (99%), as a pale-yellow oil.
The next step involved more palladium-copper-catalyzed coupling, necessary for the introduction of the two pyridyl rings. Thus, 2,5-diethynylthiophene 2 was therefore reacted with two equivalents of 4-iodopyridine, again with Pd(PPh3)2Cl2 and CuI as catalysts, in a tetrahydrofuran/triethylamine mixture, affording, after purification, the bipyridyl-derivative 3 in 84% yield as a yellow solid. This intermediate was finally converted into the desired salt through the methylation of the nitrogen atoms, with an excess of iodomethane, in CH2Cl2 at 40 °C. Compound 4, isolated by precipitation from the reaction mixture, and insoluble in CH2Cl2, was then filtrated and washed, and was obtained as an orange solid in 81% yield. Its identity and purity were confirmed by 1H- and 13C-NMR spectra, carried out in DMSO-d6, and the resulting product was pure enough to be used without further purification.
The structural determination of compound 4 could be achieved on the basis of 1D NMR spectroscopy, given its simple and symmetric structure. The 1H-NMR spectrum (Figure S1) is characterized by the presence of a sharp singlet at δ 4.34 ppm, clearly belonging to the six protons of the methyl groups attached to nitrogen atoms. As regards the aromatic part of the spectrum, the two protons in positions 3 and 4 on the thiophene ring produce a singlet at δ 7.82 ppm, while those in positions 2 and 3 of the pyridine rings, being chemically but not magnetically equivalent, give rise to a higher-order AA’XX’ spin system, yielding two multiplets, centered at δ 9.03 and 8.30 ppm, respectively.
In the 13C-NMR spectrum (Figure S2), the signal of the methyl groups on the quaternary pyridinium nitrogen can be seen at δ 48.4 ppm, whereas the acetylenic carbons produce peaks at δ 91.7 and 93.9 ppm. Regarding the aromatic rings, the peaks attributable to pyridinium carbons are the most deshielded, at δ 146.1 ppm, accounting for position 2, due to the presence of the adjacent positively charged nitrogen. The further signal at δ 129.2 ppm belongs to the carbons in position 3, while the quaternary carbons in position 4 produce the weak line at δ 137.6 ppm. Finally, the signal at δ 124.7 ppm can be ascribed to the quaternary thiophene carbons, shielded by the adjacent sp carbons of the triple bonds, while the peak found at δ 137.5 ppm is assigned to the C-H in position 3 of the sulfur-containing ring.
Similar to other already-described extended viologens [18,25,26], compound 4 proved to be soluble in H2O; its UV–visible absorption and fluorescence spectra were therefore measured in water, using a molar concentration of 2.5 × 10−4 M. The absorption spectrum is shown in Figure 1, in comparison with that of bithiophene derivative 4a (whose structure is represented in Figure 2). In both spectra, four absorption bands can be identified in the wavelength range between 190 and 500 nm; in fact, the two spectra are characterized by a markedly similar profile, given the closely related structure of the two extended viologens. The band at the lowest wavelength is the most intense one for 4, and almost superimposable to that of the other compound, with very similar λmax between the two (192 and 193 nm for 4 and 4a, respectively) and slightly different εmax (7608 M−1 cm−1 for 4 and 7988 M−1 cm−1 for 4a). The two following bands in the absorption spectrum of 4 are characterized by a lower ε and λmax, that is, 227 and 280 nm, respectively. Again, their wavelengths are very similar to those of the corresponding bands of 4a (225 nm and 275 nm), although the one at higher λ is less intense for 4 than for 4a. Finally, a large and intense band in the visible range can be observed, with λmax = 411 nm and εmax = 5232 M−1 cm−1; its wavelength, as well as its intensity, are noticeably lower, compared to those of the corresponding absorption band of bithiophene-containing 4a (λmax = 444 nm and εmax = 6658 M−1 cm−1).
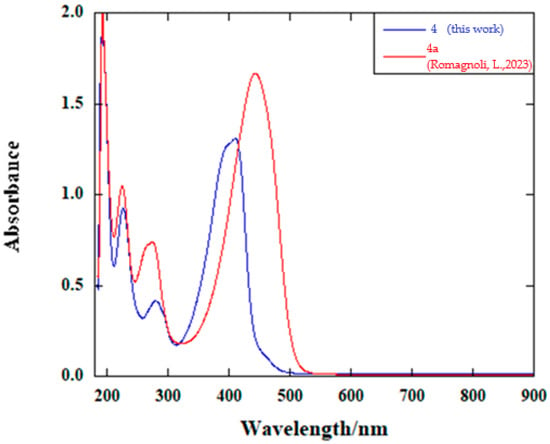
Figure 1.
UV–visible absorption spectra (H2O, c = 2.5 × 10−4 M) of 4 and 4a [18].
Figure 2.
Structure of compound 4a.
Fluorescence spectra of 4 in H2O were measured using two different wavelengths of excitation, specifically 250 nm and 300 nm, giving rise to multiple bands in the visible range, with λ of the emission maxima depending on λexc. As concerns the spectrum at λexc = 250 nm (Figure 3a), it is characterized by the presence of a large, long-tailed and convoluted band, between 420 and 700 nm, with a first maximum around 480 nm, followed by a very sharp and intense peak at λ = 501 nm; finally, another peak with a much lower intensity, almost covered by the large band, can be identified at around λ = 545 nm. The fluorescence spectrum at λexc = 300 nm (Figure 3b) also presents a similar large and asymmetric band, extending from 420 to 700 nm, convoluted with a much higher, sharp band, whose maximum could be found, in this case, at λ = 602 nm.
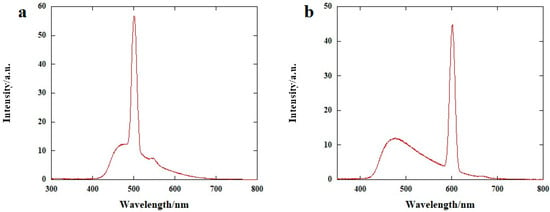
Figure 3.
Fluorescence spectra (H2O, c = 2.5 × 10−4 M) of 4, with (a) λexc = 250 nm and (b) 300 nm.
Moreover, in order to determine the redox potential of this viologen derivative, cyclic voltammetry was performed in H2O solution, using a concentration of salt 4 of 10−3 M and 0.1 M KCl as the supporting electrolyte. From the voltammogram (Figure 4), reporting V vs. SHE, a single peak can be clearly seen, both in the forward and in the reverse scan, from which a redox potential E1/2 of −0.555 V can be obtained. This is in contrast with, for example, methyl viologen, possessing two reversibly reduced states, namely the radical cation and the neutral form. A possible explanation for this finding can be attributed to the presence of the central aromatic subunit, with an electron-rich thiophene ring that could not stabilize the second electron, after the first reduction to the radical cation.
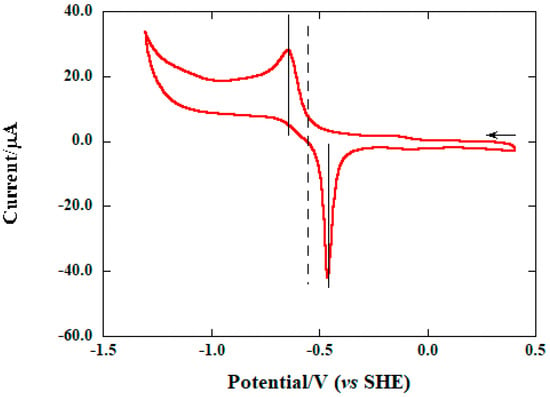
Figure 4.
Cyclic voltammetry of compound 4 in H2O solution (c = 1.0 × 10−3 M), with 0.1 M KCl as supporting electrode. Scan speed: 100 mV·s−1.
3. Materials and Methods
All the reagents were purchased from Merck and TCI and used as received unless otherwise stated. Dry solvents were distilled according to standard procedures: tetrahydrofuran was distilled over Na/benzophenone, triethylamine and diisopropylamine were distilled over KOH and dichloromethane was distilled over P2O5. Reactions and chromatographic separations were monitored by thin layer chromatography (TLC) on 0.25 mm silica gel plates (Merck Kieselgel 60 F254, Merck KGaA, Darmstadt, Germany) and revealed under a UV lamp (λ = 254 nm). Column chromatography was carried out on silica gel Merck Kieselgel 60 (Merck KGaA, Darmstadt, Germany), 0.063–0.20 mm, 70–230 mesh as a stationary phase.
1H and 13C-NMR spectra were recorded on a Bruker Avance 400 spectrometer (400 MHz) using 5 mm tubes and chloroform-d (CDCl3) and dimethyl sulfoxide-d6 (DMSO-d6) as solvents. Spectral data of compounds 1–3 were in agreement with results from the literature [27,28]. The C, H, N and S elemental analyses were performed with an EA 1110 CHNS-O elemental analyzer. The melting point of compound 4 was measured with a MEL-TEMP II apparatus using open capillary tubes and is uncorrected. The ESI-MS spectrum was acquired with a 4000 Qtrap mass spectrometer (AB Sciex, Foster City, CA, USA) equipped with an ESI source operating in the positive ion mode, and a triple quadrupole as the analyzer. The UV–Vis spectrum was measured with a Shimadzu (Kyoto, Japan) UV2600 UV–vis spectrophotometer, while fluorescence spectra were measured on a Cary Eclipse (Varian, Palo Alto, CA, USA) spectrofluorometer using SUPRASIL quartz cells (10 × 10 mm). Cyclic voltammetry measurements were carried out on a VSP BioLogic potentiostat, using a glass cell and Ag/AgCl reference electrode and carbon as the working and counter electrodes. The range of potential was between −1.5 V and 0.2 V and the scan speed of the cyclic voltammetry was 100 mV·s−1.
Synthesis of 2,5-bis((trimethylsilyl)ethynyl)thiophene (1): The following procedure is a slight modification of one already reported [29]. In a flame-dried Schlenk tube, under an argon atmosphere, 10 mL of degassed and anhydrous THF were introduced followed by 0.33 mL (2.4 mmol) of i-Pr2NH and 500 mg (1.5 mmol) of 2,5-diiodothiophene. Then, 104 mg (0.15 mmol) of Pd(PPh3)2Cl2, 30 mg (0.16 mmol) of CuI and 0.63 mL (4.5 mmol) of trimethylsilylacetylene were added and the mixture was heated to 60° C and stirred for 48 h. After that time, the reaction mixture was cooled, diluted with 10 mL of H2O and extracted with CH2Cl2 (4 × 15 mL), and then the combined organic phases were dried over anhydrous Na2SO4 and concentrated under vacuum. The crude product was purified by column chromatography in hexane, to afford 258 mg (0.93 mmol) of pure 1 (63%) as a pale-yellow solid. 1H-NMR (CDCl3, 400 MHz) δ 7.06 (s, 2H), 0.27 (s, 18H); 13C-NMR (CDCl3, 100 MHz) δ 132.6, 124.5, 100.1, 96.8, −0.2.
Synthesis of 2,5-diethynylthiophene (2): The following procedure is a slight modification of that reported in [27]. In a round-bottom flask, 258 mg (0.93 mmol) of 1 was dissolved in 12.5 mL of methanol, under argon atmosphere, and 517 mg (3.7 mmol) of K2CO3 was added. The resulting suspension was stirred overnight at room temperature, and then 10 mL of H2O was added and the solution was extracted with CH2Cl2 (4 × 15 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated under vacuum, and then the crude product was purified by column chromatography in hexane, to yield 122 mg (0.92 mmol) of pure 2 (99%) as a pale-yellow oil. 1H-NMR (CDCl3, 400 MHz) δ 7.14 (s, 2H), 3.38 (s, 2H); 13C-NMR (CDCl3, 100 MHz) δ 132.7, 123.7, 82.2, 76.2.
Synthesis of 2,5-bis(pyridin-4-ylethynyl)thiophene (3): For a different synthesis method, see reference [28]. In a flame-dried Schlenk tube, under argon atmosphere, 122 mg (0.92 mmol) of 2,5-diethynylthiophene 2 was dissolved in 10 mL of a degassed and anhydrous 1:1 THF/Et3N mixture, and then 130 mg (0.19 mmol) of Pd(PPh3)2Cl2, 18 mg (0.094 mmol) of CuI and 578 mg (2.8 mmol) of 4-iodopyridine were added. The mixture was stirred for 24 h at 60° C, then cooled, washed with a saturated NH4Cl solution, and the aqueous phase was washed with ethyl acetate (3 × 15 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated under vacuum, and then the crude product was purified by column chromatography (1:1 → 4:6 hexane/ethyl acetate), to afford 222 mg (0.78 mmol) of pure 3 as a yellow solid (84%). 1H-NMR (CDCl3, 400 MHz) δ 8.66 (m, 4H), 7.40 (m, 4H), 7.27 (s, 2H); 13C-NMR (CDCl3, 100 MHz) δ 149.8, 133.2, 130.6, 125.3, 124.6, 91.9, 86.4.
Synthesis of 4,4’-(thiophene-2,5-diylbis(ethyne-2,1-diyl))bis(1-methyl-1-pyridinium) iodide (4): In a flame-dried Schlenk tube, 222 mg (0.78 mmol) of 3 was dissolved in 15 mL of anhydrous CH2Cl2 and 3.5 mL (56 mmol) of CH3I was added under an argon atmosphere. The mixture was stirred at 40° C for 24 h, and then cooled to room temperature and filtered under vacuum; the solid was washed with portions of CH2Cl2 and dried for 1 h under suction, to yield 358 mg (0.63 mmol) of pure product 4, as an orange solid (81%). 1H-NMR (DMSO-d6, 400 MHz) δ 9.03 (m, 4H), 8.30 (m, 4H), 7.82 (s, 2H), 4.34 (s, 6H); 13C-NMR (DMSO-d6, 100 MHz) δ 146.1, 137.6, 137.5, 129.2, 124.7, 93.9, 91.7, 48.4. Melting point: 228–230 °C (dec.). Elemental analysis, calculated: C 42.13; H 2.83; N 4.91; S 5.62. Found: C 40.14; H 2.57; N 4.46; S 5.83. MS (+ESI), m/z: [M+H]+: 571.3.
4. Conclusions
An extended viologen with a very simple structure, characterized by the presence of a central thiophene ring and two triple bonds as linking moieties to the quaternary pyridinium rings, was obtained with a straightforward four-step synthesis in 42% overall yield. This salt was characterized by UV–visible absorbance and fluorescence spectroscopy, exhibiting very effective absorption and emission of light, especially in the visible range, similar to the related bithiophene-containing analogue. Moreover, the electrochemical behavior of this viologen was investigated by cyclic voltammetry, which showed the presence of only one reversibly reduced state. The easy synthesis and interesting properties of this compound make it appealing for a number of optoelectronic applications.
Supplementary Materials
The following supporting information can be downloaded: Figure S1: 1H-NMR spectrum of the title compound; Figure S2: 13C-NMR spectrum of the title compound.
Author Contributions
Conceptualization, A.D., A.L. and L.R; methodology, L.R. and A.D.; formal analysis, L.R.; investigation, L.R.; writing—original draft preparation, L.R.; writing—review and editing, A.D.; supervision, A.D. and A.L.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to thank Sapienza Università di Roma for financial support (project no AR12117A898DA49C).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors wish to thank Marco Agostini for performing cyclovoltammetry measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Madaan, P.; Tyagi, V.K. Quaternary Pyridinium Salts: A Review. J. Oleo Sci. 2008, 57, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Sowmiah, S.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Pyridinium salts: From synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Yake, A.; Corder, T.; Moloy, K.; Coope, T.; Taylor, C.; Hung, M.; Peng, S. Fluorinated pyridinium and ammonium cationic surfactants. J. Fluorine Chem. 2016, 187, 46–55. [Google Scholar] [CrossRef]
- Tamilarasan, R.; Ganesan, K.; Subramani, A.; Ali, L.B.; Alam, M.M.; Mohammed, A. Synthesis, Characterization, Pharmacogenomics, and Molecular Simulation of Pyridinium Type of Ionic Liquids and Their Applications. ACS Omega 2023, 8, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Aparicio, J.L.; Meas, Y.; Chapman, T.W.; Trejo, G.; Ortega, R.; Chainet, E. Electrodeposition of zinc in the presence of quaternary ammonium compounds from alkaline chloride bath. J. Appl. Electrochem. 2015, 45, 67–78. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, Q.; Liu, J.; He, Q.; Gong, C.; Tang, Q.; Shen, W. Electrochromic materials containing pyridinium salt and benzoate moieties with dual-colored and long-life performance. Sol. Energy Mater. Sol. Cells 2022, 240, 111712. [Google Scholar] [CrossRef]
- Parashar, R.K.; Kandpal, S.; Pal, N.; Manna, D.; Pal, B.N.; Kumar, R.; Mondal, P.C. Coexistence of Electrochromism and Bipolar Nonvolatile Memory in a Single Viologen. ACS Appl. Mater. Interfaces 2023, 15, 51527–51537. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; He, Y.; Han, J.-M. Helical perylene diimide self-assembly with a redox-active molecular switch applied to humidity sensing. J. Mater. Chem. A 2022, 10, 18363–18373. [Google Scholar] [CrossRef]
- Turgut, K.; Altinisik, S.; Yanalak, G.; Koyuncu, S.; Patir, I.H. Enhanced Photocatalytic Hydrogen Evolution by Star-Shaped Viologen-Sensitized TiO2 Nanoparticles. ACS Appl. Nano Mater. 2023, 6, 20173–20182. [Google Scholar] [CrossRef]
- Ma, T.; Liu, L.; Wang, J.; Lu, Y.; Chen, J. Charge Storage Mechanism and Structural Evolution of Viologen Crystals as the Cathode of Lithium Batteries. Angew. Chem. Int. Ed. 2020, 59, 11533–11539. [Google Scholar] [CrossRef]
- Li, H.; Fan, H.; Hu, B.; Hu, L.; Chang, G.; Song, J. Spatial Structure Regulation: A Rod-Shaped Viologen Enables Long Lifetime in Aqueous Redox Flow Batteries. Angew. Chem. Int. Ed. 2021, 60, 26971–26977. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.J.; Ferris, D.P.; Vermeulen, N.A.; Henkelis, J.J.; Popovs, I.; Juríček, M.; Barnes, J.C.; Schneebeli, S.T.; Stoddart, J.F. Cooperative Reactivity in an Extended-Viologen-Based Cyclophane. J. Am. Chem. Soc. 2016, 138, 3667–3670. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Fan, Y.; Li, W.-X.; Zhang, X.; Liang, R.-R.; Lin, F.; Zhan, T.-G.; Cui, J.; Liu, L.-J.; Zhao, X.; et al. Viologen derivatives with extended π-conjugation structures: From supra-/molecular building blocks to organic porous materials. Chin. Chem. Lett. 2020, 31, 1757–1767. [Google Scholar] [CrossRef]
- Barravecchia, L.; Blanco-Gómez, A.; Neira, I.; Skackauskaite, R.; Vila, A.; Rey-Rico, A.; Peinador, C.; García, M.D. “Vermellogens” and the Development of CB [8]-Based Supramolecular Switches Using pH-Responsive and Non-Toxic Viologen Analogues. J. Am. Chem. Soc. 2022, 144, 19127–19136. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, L.; D’Annibale, A.; Latini, A. 4,4′-([2,2′-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide. Molbank 2023, 2023, M1733. [Google Scholar] [CrossRef]
- Iftikhar, R.; Khan, F.Z.; Naeem, N. Recent synthetic strategies of small heterocyclic organic molecules with optoelectronic applications: A review. Mol. Divers. 2023, 28, 271–307. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; He, C.; Liu, C.; Fan, Y.; Zhao, C.; Zhao, C.; Su, W.; Dappe, Y.J.; Yang, L. Oligothiophene molecular wires at graphene-based molecular junctions. Phys. Chem. Chem. Phys. 2021, 23, 21163–21171. [Google Scholar] [CrossRef]
- Chemek, M.; Braiek, M.B.; Mabrouk, A.; Wazzan, N.; Mansour, A.B.; Hafiane, O.; Kamel, A. Optoelectronic properties of a new luminescent-synthesized organic material based on carbazole and thiophene rings for a new generation of OLEDs devices: Experimental investigations and DFT modeling. J. Mater. Sci. Mater. Electron. 2023, 34, 1706. [Google Scholar] [CrossRef]
- Chen, H.; Kan, B.; Wang, P.; Feng, W.; Li, L.; Zhang, S.; Chen, T.; Yang, Y.; Duan, T.; Yao, Z.; et al. Terminally Chlorinated and Thiophene-linked Acceptor-Donor-Acceptor Structured 3D Acceptors with Versatile Processability for High-efficiency Organic Solar Cells. Angew. Chem. Int. Ed. 2023, 62, e2023079. [Google Scholar]
- Haya, G.; Runsewe, D.O.; Otakpor, M.U.; Pohlman, G.E.; Towne, A.; Betancourt, T.; Irvin, J.A. Functionalized Thiophene-Based Aptasensors for the Electrochemical Detection of Mucin-1. ACS Appl. Polym. Mater. 2023, 5, 1208–1218. [Google Scholar] [CrossRef]
- Karak, M.; Barbosa, L.C.A.; Hargaden, G.C. Recent mechanistic developments and next generation catalysts for the Sonogashira coupling reaction. RSC Adv. 2014, 4, 53442–53466. [Google Scholar] [CrossRef]
- Romagnoli, L.; D’Annibale, A.; Blundo, E.; Polimeni, A.; Cassetta, A.; Chita, G.; Panetta, R.; Ciccioli, A.; Latini, A. Synthesis, Structure, and Characterization of 4,4′-(Anthracene-9,10-diylbis(ethyne-2,1-diyl))bis(1-methyl-1-pyridinium) Bismuth Iodide (C30H22N2)3Bi4I18, an Air, Water, and Thermally Stable 0D Hybrid Perovskite with High Photoluminescence Efficiency. Cryst. Growth Des. 2022, 22, 7426–7433. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, L.; D’Annibale, A.; Latini, A. 4,4′,4″-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide. Molbank 2023, 2023, M1742. [Google Scholar] [CrossRef]
- Neenan, T.X.; Whitesides, G.M. Synthesis of high carbon materials from acetylenic precursors. Preparation of aromatic monomers bearing multiple ethynyl groups. J. Org. Chem. 1988, 53, 2489–2496. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Friščić, T.; MacGillivray, L.R. Enforced Face-to-Face Stacking of Organic Semiconductor Building Blocks within Hydrogen-Bonded Molecular Cocrystals. J. Am. Chem. Soc. 2006, 128, 2806–2807. [Google Scholar] [CrossRef]
- Lu, P.; Lam, J.W.Y.; Liu, J.; Jim, C.K.W.; Yuan, W.; Chan, C.Y.K.; Xie, N.; Hu, Q.; Cheuk, K.K.L.; Tang, B.Z. Regioselective Alkyne Polyhydrosilylation: Synthesis and Photonic Properties of Poly(silylenevinylene)s. Macromolecules 2011, 44, 5977–5986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).