Abstract
The reaction of sodium 2,2-dicyanoethene-1,1-bis(thiolate) with bromine (2 equiv.) in CCl4 gave 3,5-dibromoisothiazole-3-carbonitrile and 5,5′-thiobis(3-bromoisothiazole-4-carbonitrile) in 7% and 18% yields, respectively. The latter novel compound was fully characterized.
1. Introduction
Heterocycle sulfides are a particularly important group of compounds with numerous examples of biologically useful compounds such as the immunosuppressant Azathioprine 1 [1,2,3], the antibacterial drug meropenem 2 [4,5,6] and the herbicide pyriftalid 3 [7,8,9] (Figure 1). Focusing on isothiazole sulfides, there are several examples of biologically useful compounds such as the 4-cyanoisothiazole 4 that has shown antiviral activity against polio [10,11,12] and dithiine 5 which is active as an antifungal agent [13] (Figure 1).
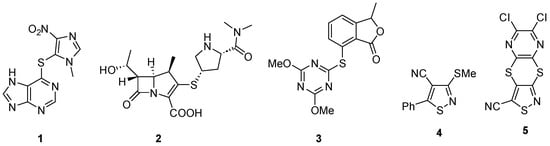
Figure 1.
Biologically active isothiazole carbonitriles.
Isothiazoles are five-membered heterocycles that have found uses as agrochemicals [14], pharmaceuticals [15] and dyes [16]. Their applications, chemistry and synthesis have been reviewed [17,18,19]. Examples of biologically useful isothiazoles are the fungicide isotianil (Stout®) [20,21], active against rice blast, and the antibacterial drug sulfasomizole [22,23].
Our interest in isothiazoles focuses on their preparation from 1,2,3-dithiazoles 6 by treatment with gaseous HCl or HBr [24,25] (Scheme 1), halide or alkylamines [26]. Moreover, we were interested in the investigation of the chemistry of halo and cyano-substituted isothiazoles. Halogen atoms in the C-5 position were substituted by carbon nucleophiles in Suzuki [27], Stille and Sonogashira couplings [28] (Scheme 1), while the coupling chemistry of the C-3 [28] and later the C-4 positions [29] was also investigated. Interestingly, the isothiazole C-4 cyano group has been converted to a bromo group via a Hunsdiecker strategy or to an iodo group via a Hoffmann and Sandmeyer strategy [29].
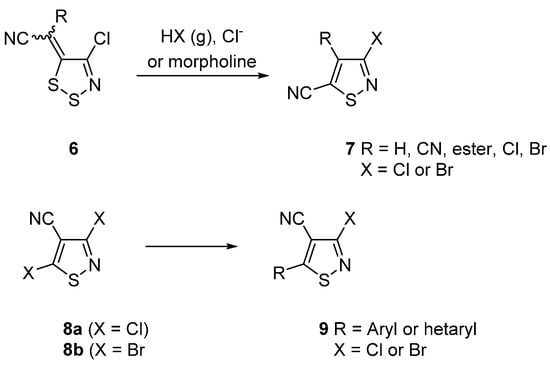
Scheme 1.
Route to isothiazole-5-carbonitriles 7 from dithiazoles 6 and coupling chemistry of 3-haloisothiazoles 8.
An important isothiazole scaffold that we required in the course of our investigations is 3,5-dibromoisothiazole-3-carbonitrile (8b) (Scheme 1). The synthesis of this highly functionalized isothiazole that offers many options for functional group modifications is reported in the literature [30,31].
Herein, we report our findings in performing this reaction that led to the isolation of 5,5′-thiobis(3-bromoisothiazole-4-carbonitrile) (10). The formation of this compound through the treatment of 3,5-dibromoisothiazole-4-carbonitrile with sodium thiocyanate is mentioned in the patent literature [31], but no yield or characterization data are described.
The preparation of sulfide 10 differs from most reported methods of preparation of isothiazole sulfides that commonly involve the nucleophilic aromatic substitution of halo-isothiazoles with thiols [32] or palladium-catalyzed C-S coupling [33].
2. Results and Discussion
The reaction of sodium 2,2-dicyanoethene-1,1-bis(thiolate) (11) with bromine (2 equiv.) in CCl4 at ca. 55 °C, by a modification of the reported method [30,31] gave, after workup and chromatography, 3,5-dibromoisothiazole-4-carbonitrile (8b) and 5,5′-thiobis(3-bromoisothiazole-4-carbonitrile) (10) in 7% and 18% yields, respectively (Scheme 2).
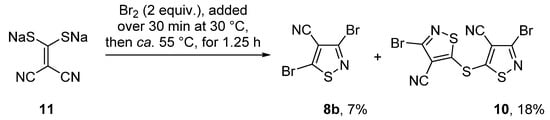
Scheme 2.
Synthesis of 5,5′-thiobis(3-bromoisothiazole-4-carbonitrile) (10).
Product 10 was isolated as yellow plates, mp 141–142 °C (from PhH). UV-vis spectroscopy in dichloromethane supported an intact isothiazole ring [λmax(DCM) 279 nm, log ε 4.18], while FTIR spectroscopy showed the presence of a ν(C≡N) stretch at 2334 cm−1. Mass spectrometry revealed a molecular ion (MH+) peak of m/z 407 (38%) along with a MH+ + 2 isotope peak at 408 (85%) and a MH+ + 4 at 411 (54%) that supported the presence of two bromine atoms. 13C NMR spectroscopy showed the presence of four quaternary carbon resonances (see Supplementary Information), while a correct elemental analysis (CHN) was obtained for the molecular formula C8Br2N4S3. The multifunctional nature of isothiazole 10 makes it a potentially useful synthetic scaffold.
Mechanistically, we attribute the formation of sulfide 10 to a reaction of product 8b with a source of nucleophilic sulfur. The initial displacement of the 5-bromide should lead to 3-bromo-5-mercaptoisothiazole-4-carbonitrile 12, which could condense with another molecule of isothiazole 8b to yield product 10 (Scheme 3). Interestingly, sulfide 13, which is the chloro analogue of sulfide 10, can be prepared by the reaction of 3,5-dichloroisothiazole-4-carbonitrile 8a with either CuCN (1 equiv.), NaSCN (1 equiv.) or Na2S (0.5 equiv.) [34] (Scheme 3). In the latter two methods, it is clear that nucleophilic sulfur is involved similarly to our proposal for the formation of sulfide 10.
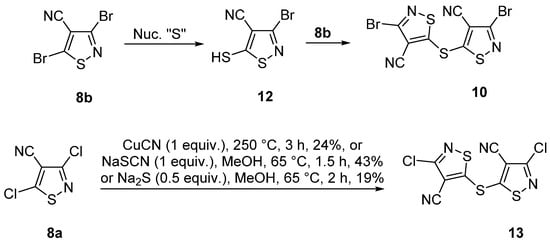
Scheme 3.
Origins of sulfide 10 and reported syntheses of 5,5′-thiobis(3-chloroisothiazole-4-carbonitrile) (13).
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass-backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler—Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a Perkin-Elmer Lambda-25 UV-vis spectrophotometer (Perkin-Elmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA), and strong, medium and weak peaks are represented by s, m and w, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 machine [at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA)]. Deuterated solvents were used for homonuclear lock and the signals were referred to with the deuterated solvent peaks. Attached proton test (APT) NMR studies were used for the assignment of the 13C peaks as CH3, CH2, CH and Cq (quaternary). MALDI-TOF mass spectra were recorded on a Bruker Autoflex III Smartbeam instrument. Sodium 2,2-dicyanoethene-1,1-bis(thiolate) (11) [30] was prepared according to the literature procedure.
5,5′-Thiobis(3-bromoisothiazole-4-carbonitrile) (10)
A suspension of sodium 2,2-dicyanoethene-1,1-bis(thiolate) (11) (223.3 g, 1.20 mol) in CCl4 (2.4 L) in a 5 L round bottom flask fitted with a mechanical stirrer, thermometer and condenser was added dropwise to bromine (123 mL, 2.40 mol) under stirring over 30 min. The temperature of the mixture rose to ca. 30 °C during the addition. The mixture was then heated in a heating mantle to ca. 55 °C and stirred for a further 1.25 h. The mixture was then filtered through a pad of silica to remove insoluble matter and washed with DCM (a total of 2 L); the filtrate was then adsorbed onto silica and chromatographed (n-hexane/DCM, 80:20) to give 3,5-dibromoisothiazole-4-carbonitrile (8b) (23.16 g, 7%) as colorless needles, mp 98–99 °C (sublimed, lit. [28] 98–98.5 °C); Rf 0.28 (n-hexane/DCM, 80:20); vmax/cm−1 2232m (C≡N), 1488s, 1369m, 1351w, 1313s, 1207w, 1071m, 965m, 955m, 935w, 912w, 803s, 766m, identical to the one reported [30]. A further elution (n-hexane/DCM, 50:50) gave the title compound 10 (44.87 g, 18%) as yellow plates, mp 141–142 °C, (from PhH); Rf 0.33 (n-hexane/DCM, 50:50); (found: C, 23.31; H, 0; N, 13.58. C8Br2N4S3 requires C, 23.54; H, 0; N, 13.73%); λmax(DCM)/nm 230 (4.01), 279 (4.18), 319 inf (3.41); vmax/cm−1 2234m (C≡N), 1478m, 1346w, 1319s, 1086m, 949m, 937m, 824m, 812s; δC(125 MHz; CDCl3) 163.3 (Cq), 140.2 (Cq), 116.7 (Cq), 109.8 (Cq); m/z (MALDI-TOF) 411 (MH+ + 4, 54%), 409 (MH+ + 2, 85), 407 (MH+, 38), 402 (64), 329 (M-Br + 2, 100), 327 (M-Br, 63).
Supplementary Materials
The following supporting information can be downloaded online: mol file, 13C NMR and IR spectra.
Author Contributions
A.S.K. and P.A.K. conceived the experiments; A.S.K. designed the experiments; A.S.K. wrote the paper; A.S.K. and P.A.K. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cyprus Research Promotion Foundation, grant numbers ΣTPATHII/0308/06, NEKYP/0308/02 ΥΓEIA/0506/19 and ΕΝΙΣX/0308/83.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: The State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd., Medisell Ltd. and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bandaru, S.S.M.; Bhilare, S.; Cardozo, J.; Chrysochos, N.; Schulzke, C.; Sanghvi, Y.S.; Gunturu, K.C.; Kapdi, A.R. Pd/PTABS: Low-Temperature Thioetherification of Chloro(hetero)arenes. J. Org. Chem. 2019, 84, 8921–8940. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Pasadhika, S.; Kempen, J.H.; Newcomb, C.W.; Liesegang, T.L.; Pujari, S.S.; Rosenbaum, J.T.; Thorne, J.E.; Foster, C.S.; Jabs, D.A.; Levy-Clarke, G.A.; et al. Azathioprine for Ocular Inflammatory Diseases. Am. J. Ophthalm. 2009, 148, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Neera, T.; Shailendra, S.K.; Kishore, M.B.; Sarawati, R. Process for the Preparation of Carbapemen Compounds. WO Patent 2010013223A1, 4 February 2010. [Google Scholar]
- Edwards, J.R. Meropenem: A microbiological overview. J. Antimicrob. Chemother. 1995, 36, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.M.; Lyseng-Williamson, K.A.; Keam, S.J. Meropenem. Drugs 2008, 68, 803–838. [Google Scholar] [CrossRef] [PubMed]
- Luethy, C.; Zondler, H.; Rapold, T.; Seifert, G.; Urwyler, B.; Heinis, T.; Steinruecken, H.C.; Allen, J. 7-(4,6-Dimethoxypyrimidinyl)oxy- and -thiophthalides as novel herbicides: Part 1. CGA 279 233: A new grass-killer for rice. Pest Manag. Sci. 2001, 57, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Behavior of pyriftalid in soil and its phytotoxic activity on Echinochloa oryzoides seedlings emerging from various soil depths. Weed Biol. Manag. 2010, 10, 249–255. [Google Scholar] [CrossRef]
- Li, Z.; Yang, A.; Xia, W.; Qiu, P.; Zhang, F. An Innovative and Concise Approach to Synthesize Pyriftalid. Org. Prep. Proced. Int. 2017, 49, 382–388. [Google Scholar] [CrossRef]
- Garozzo, A.; Stivala, A.; Tempera, G.; Castro, A. Antipoliovirus activity and mechanism of action of 3-methylthio-5-phenyl-4-isothiazolecarbonitrile. Antivir. Res. 2010, 88, 325–328. [Google Scholar] [CrossRef]
- Cutri, C.C.C.; Garozzo, A.; Siracusa, M.A.; Sarva, M.C.; Tempera, G.; Geremia, E.; Pinizzotto, M.R.; Guerrera, F. Synthesis and antiviral activity of a new series of 4-isothiazolecarbonitriles. Bio. Med. Chem. 1998, 6, 2271–2280. [Google Scholar] [CrossRef]
- Cutri, C.C.C.; Garozzo, A.; Siracusa, M.A.; Sarva, M.C.; Tempera, G.; Geremia, E.; Pinizzotto, M.R.; Guerrera, F. Synthesis of new 3,4,5-trisubstituted isothiazoles as effective inhibitory agents of enteroviruses. Bio. Med. Chem. 1999, 7, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Soldato, P.D.; Benedini, F. Nitrate Salts of Antimicrobial Agents. U.S. Patent 2003105066A1, 5 June 2023. [Google Scholar]
- Baumann, E.; Schieweck, F.; Von Deyn, W.; Blasco, T.; Blettner, C.; Mueller, B.; Gewehr, M.; Grammenos, W.; Grote, T.; Gypser, A.; et al. 5-(2-Arylacetamido)isothiazole Compounds II. WO Patent 2,005,040,143, 6 May 2005. [Google Scholar]
- Gunic, E.; Appleby, T.; Zhong, W.; Yan, S.; Lai, H.C. Cycloalkl, Aryl and Heteroaryl Amino Isothiazoles for the Treatment of Hepatitis C virus. U.S. Patent 2,006,217,390, 28 September 2006. [Google Scholar]
- Kanitz, A.; Hartmann, H. Preparation and Characterization of Bridged Naphthoxazinium Salts. Eur. J. Org. Chem. 1999, 4, 923–930. [Google Scholar] [CrossRef]
- Clerici, F.; Gelmi, M.L.; Pellegrino, S. Comprehensive Heterocyclic Chemistry III; Joule, J., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, Chapter 4.05; pp. 545–633. [Google Scholar]
- Pain, D.L.; Peart, B.J.; Woordridge, K.R.H. Comprehensive Heterocyclic Chemistry I; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; Volume 6, p. 131. [Google Scholar]
- Chapman, R.F.; Peart, B.J. Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; Volume 3, p. 319. [Google Scholar]
- Bi, F.; Didiuk, M.T.; Guzman-Perez, A.; Griffith, D.A.; Liu, K.K.C.; Walker, D.P.; Zawistoski, M.P. Thieno[2,3-d]pyrimidin-4(3H)-one, Isozazolo[5,4-d]pyridin-4(5H)-one and Isothiazolo[5,4-d]pyrimidin-4(5H)-one Derivatives as Calcium Receptor Antagonists. WO Patent 2009/1214, 31 December 2008. [Google Scholar]
- Portz, K.; Casanova, F.; Jordine, A.; Bohnert, S.; Mehl, A.; Portz, D.; Schaffrath, U. Wheat blast caused by Magnaporthe oryzae pathovar Triticum is efficiently controlled by the plant defence inducer isotianil. J. Plant Dis. Protect. 2021, 128, 249–259. [Google Scholar] [CrossRef]
- Grammenos, W.; Boudet, N.; Muller, B.; Quintero, P.M.A.; Escibaro, C.A.; Lauterwasser, E.M.W.; Lohmann, J.K.; Grote, T.; Kretscher, M.; Feur, M. Substituted 1,4-Dithiine Derivatives and Their Use as Fungicides. WO Patent 2015150135A1, 8 October 2015. [Google Scholar]
- Nagaraju, C.; Ashok, S.H.; Shamanth, S.; Nagarakere, S.C.; Sunilkumar, M.P.; Subbegowda, R.K.; Mantelingu, K. A novel and facile synthesis of 3,5-Disubstituted isothiozoles under metal free conditions using acetophenones and dithioesters. Synth. Comm. 2020, 50, 2647–2654. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2013, 4, 7735–7748. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Rees, C.W. Reactions of 1,2,3-dithiazoles with halogenated malononitriles. J. Chem. Soc. Perkin Trans. 1 2002, 10, 1236–1241. [Google Scholar] [CrossRef]
- Emayan, K.; English, R.F.; Koutentis, P.A.; Rees, C.W. New routes to benzothiophenes, isothiazoles and 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1997, 22, 3345–3349. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Rees, C.W. Regiospecific Suzuki coupling of 3,5-dichloroisothiazole-4-carbonitrile. Org. Biomol. Chem. 2003, 1, 2900–2907. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A. New regiospecific isothiazole C–C coupling chemistry. Org. Biomol. Chem. 2006, 4, 3681–3693. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A. 3,4,5-Triarylisothiazoles via C–C coupling chemistry. Org. Biomol. Chem. 2007, 5, 1381–1390. [Google Scholar] [CrossRef]
- Hatchard, W.R. The Synthesis of Isothiazoles. I. 3,5-Dichloro-4-isothiazolecarbonitrile and Its Derivatives. J. Org. Chem. 1964, 29, 660–665. [Google Scholar] [CrossRef]
- Hatchard, W.R. 5, 5-thiobis(isothiazole) Compounds and Their Production. U.S. Patent 3155679A, 3 November 1964. [Google Scholar]
- De, S.K.; Stebbins, J.L.; Chen, L.-H.; Riel-Mehan, M.; Machleidt, T.; Dahl, R.; Yuan, H.; Emdadi, A.; Barile, E.; Chen, V.; et al. Design, Synthesis, and Structure−Activity Relationship of Substrate Competitive, Selective, and in Vivo Active Triazole and Thiadiazole Inhibitors of the c-Jun N-Terminal Kinase. J. Med. Chem. 2009, 52, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, J.; Kuno, H.; Nara, H.; Oki, H.; Sato, K. Heterocyclic amide compound and use thereof as an MMP-13 inhibitor. WO Patent 2005105760A1, 10 November 2005. [Google Scholar]
- Lee, F.T.; Li, B.W.; Volpp, G.P. Bis(3-chloro-4-cyanoisothiazol-5-yl) sulfide. J. Heterocycl. Chem. 1970, 7, 941–942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).