The Synthesis and Structure of Scandium Dichloride of Sterically Demanding Aminopyridinato Ligands
Abstract
1. Introduction
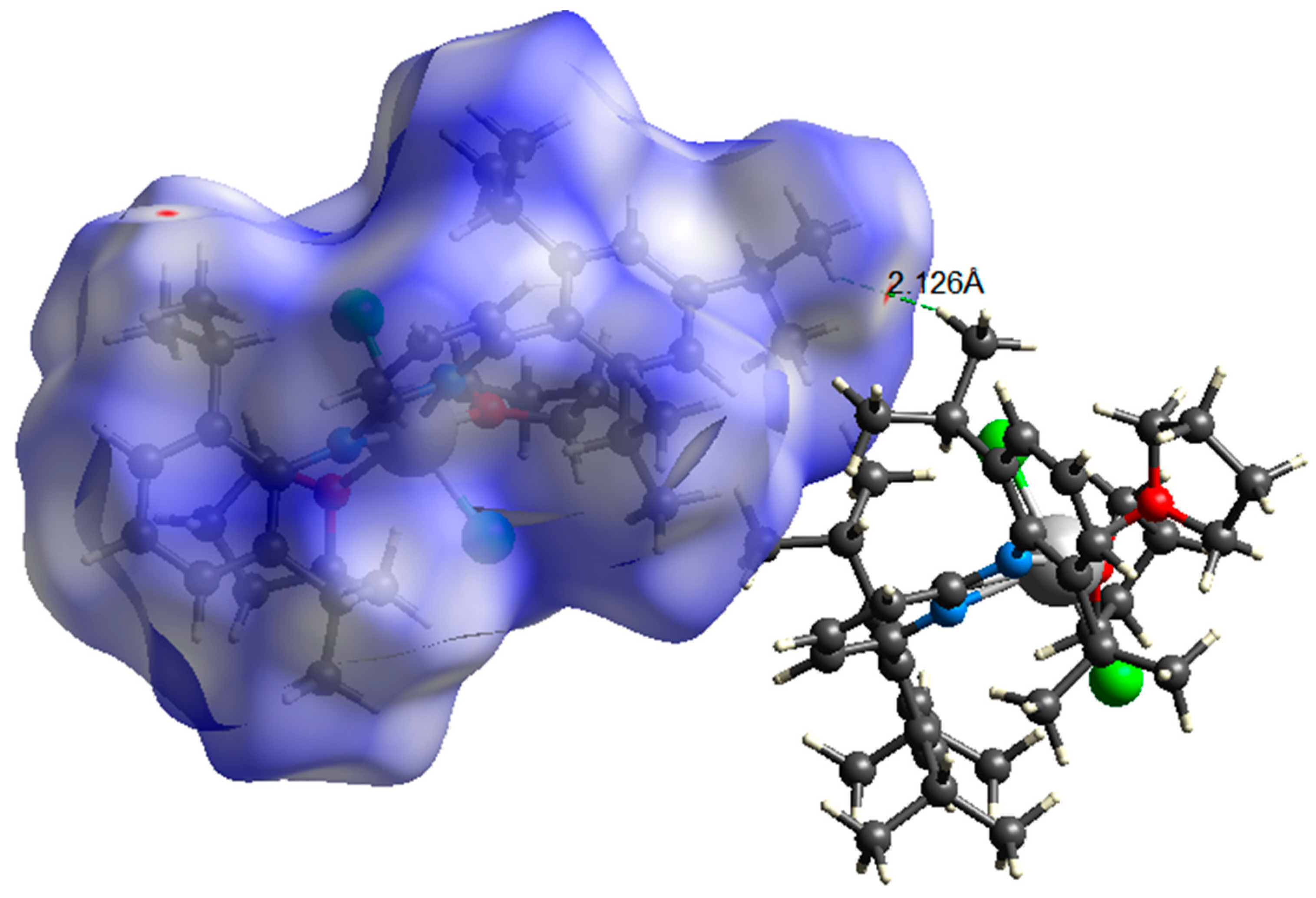
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kempe, R. The Strained η2-NAmido−NPyridine Coordination of Aminopyridinato Ligands. Eur. J. Inorg. Chem. 2003, 2003, 791–803. [Google Scholar] [CrossRef]
- Noor, A. Coordination chemistry of bulky aminopryridinates with main group and transition metals. Top. Curr. Chem. 2021, 379, 6. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.M.; Schareina, T.; Tok, O.; Kempe, R. Lithium and potassium amides of sterically demanding aminopyridines. Eur. J. Inorg. Chem. 2004, 2004, 3297–3304. [Google Scholar] [CrossRef]
- Noor, A. Crystallographic Evidence of η1-Coordination of Bulky Aminopyridine in Halide-Containing Iron (II) Complexes. Crystals 2022, 12, 697. [Google Scholar] [CrossRef]
- Noor, A.; Wagner, F.R.; Kempe, R. Metal–Metal Distances at the Limit: A Coordination Compound with an Ultrashort Chromium–Chromium Bond. Angew. Chem. Int. Ed. 2008, 47, 7246–7249. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Glatz, G.; Müller, R.; Kaupp, M.; Demeshko, S.; Kempe, R. Carboalumination of a chromium–chromium quintuple bond. Nat. Chem. 2009, 1, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Qayyum, S.; Schwarz, S.; Dietel, T.; Kempe, R. Formation of a dimeric tungsten(i) complex via C–H activation. Dalton Trans. 2020, 49, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Kempe, R. Rare Earth Polymerization Catalysts Supported by Bulky Aminopyridinato Ligands. Z. Anorg. Allg. Chem. 2010, 636, 2135–2147. [Google Scholar] [CrossRef]
- Dietel, T.; Lukas, F.; Kretschmer, W.P.; Kempe, R. Elongation and branching of α-olefins by two ethylene molecules. Science 2022, 375, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, W.P.; Meetsma, A.; Hessen, B.; Scott, N.M.; Qayyum, S.; Kempe, R. Lanthanum dibromide complexes of sterically demanding aminopyridinato and amidinate ligands. Z. Anorg. Allg. Chem. 2006, 632, 1936–1938. [Google Scholar] [CrossRef]
- Noor, S.Q.A.; Glatz, G.; Kempe, R.Z. Attempted Reduction of Divalent Rare Earth Iodo Aminopyridinates. Z. Anorg. Allg. Chem. 2009, 635, 2455–2458. [Google Scholar]
- Noor, A.; Kretschmer, W.; Kempe, R. Synthesis and Structure of Trialkyltantalum Complexes Stabilized by Aminopyridinato Ligands. Eur. J. Inorg. 2006, 2006, 2683–2689. [Google Scholar] [CrossRef]
- Qayyum, S. Syntheses and structural characterization of tris-and tetrakis (aminopyridinate) complexes of lanthanum. Inorg. Chim. Acta 2024, 559, 121768. [Google Scholar] [CrossRef]
- Glatz, G.; Demshko, S.; Motz, G.; Kempe, R. First Row Transition Metal Aminopyridinates–The Missing Complexes. Eur. J. Inorg. 2009, 2009, 1385–1392. [Google Scholar] [CrossRef]
- Döring, C.; Kretschmer, W.P.; Bauer, T.; Kempe, R. Scandium aminopyridinates: Synthesis, structure and isoprene polymerization. Eur. J. Inorg. Chem. 2009, 2009, 4255–4264. [Google Scholar] [CrossRef]
- Qayyum, S.; Skvortsov, G.G.; Fukin, G.K.; Trifonov, A.A.; Kretschmer, W.P.; Döring, C.; Kempe, R. Intramolecular C–H Bond Activation by Lanthanoid Complexes Bearing a Bulky Aminopyridinato Ligand. Eur. J. Inorg. Chem. 2010, 2010, 248–257. [Google Scholar] [CrossRef]
- Guillaume, S.M.; Schappacher, M.; Scott, N.M.; Kempe, R. Di- and trivalent rare earth complexes stabilized by sterically demanding aminopyridinato ligands as initiators in ring-opening polymerization reactions of ϵ-caprolactone. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3611–3619. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; University of Western Australia: Perth, WA, Australia, 2017. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115−119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX Suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837−838. [Google Scholar] [CrossRef]




| Empirical Formula | C40H59Cl2N2O2Sc |
|---|---|
| Formula weight | 715.75 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a [Å] | 12.4441(8) |
| b [Å] | 22.9975(10) |
| c [Å] | 13.9971(8) |
| α [deg] | |
| β [deg] | 92.297(5) |
| γ [deg] | |
| V, [Å3] | 4002.5(4) |
| Crystal size, [mm3] | 0.3 × 0.3 × 0.2 |
| ρcalcd, [g cm−3] | 1.188 |
| µ, [mm−1] (Mo Kα) | 0.351 |
| T, [K] | 193(2) |
| 2θ range, [deg] | 3.40–51.46 |
| No. of reflections (unique) | 7530 |
| No. of reflections obs. [I > 2σ (I)] | 6557 |
| No. of parameters | 424 |
| wR2 (all data) | 0.0966 |
| R-value [I > 2σ (I)] | 0.0376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qayyum, S. The Synthesis and Structure of Scandium Dichloride of Sterically Demanding Aminopyridinato Ligands. Molbank 2024, 2024, M1805. https://doi.org/10.3390/M1805
Qayyum S. The Synthesis and Structure of Scandium Dichloride of Sterically Demanding Aminopyridinato Ligands. Molbank. 2024; 2024(2):M1805. https://doi.org/10.3390/M1805
Chicago/Turabian StyleQayyum, Sadaf. 2024. "The Synthesis and Structure of Scandium Dichloride of Sterically Demanding Aminopyridinato Ligands" Molbank 2024, no. 2: M1805. https://doi.org/10.3390/M1805
APA StyleQayyum, S. (2024). The Synthesis and Structure of Scandium Dichloride of Sterically Demanding Aminopyridinato Ligands. Molbank, 2024(2), M1805. https://doi.org/10.3390/M1805






