Abstract
A simplified synthesis of the title compound is reported and its 1H and 13C NMR data are fully assigned including determination of H–H and C–H coupling constants. Its X-ray structure has been determined for the first time. NMR data are also presented for the oxygen analogue.
1. Introduction
The simple compound benzo[d][1,3,2]dioxathiole 2-oxide or “catechol sulfite” 1 was first reported in 1894 []. It shows a rather interesting pattern of reactivity upon pyrolysis (Scheme 1), undergoing sequential loss of sulfur monoxide and carbon monoxide to generate cyclopentadienone, isolated as its stable Diels–Alder dimer 2 [], and this reaction has been developed into a convenient undergraduate laboratory experiment to illustrate the use of flash vacuum pyrolysis (FVP) []. The corresponding sulfur analogue benzo[d][1,2,3]oxadithiole 2-oxide 3 has only been mentioned in a single paper [], where its gas phase pyrolysis was used to generate cyclopentadienethione 4, detected by photoelectron spectroscopy, but no stable product was isolated in this case.
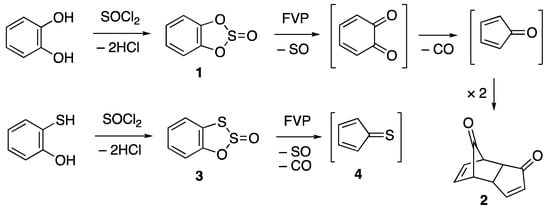
Scheme 1.
Synthesis and pyrolytic behaviour of compounds 1 and 3.
The extent of previous characterisation of the fundamental compounds 1 and 3 by NMR is rather limited, with just a single chemical shift reported for both 1 [] and 3 [] in the 1H NMR spectra and, while 13C NMR chemical shifts are listed for 3 [] without assignment, we are not aware of any published 13C NMR data for 1.
In the context of renewed studies on the FVP behaviour of 3, we have reinvestigated these compounds, and in this paper, we report a full NMR study of 1 and 3 as well as the X-ray structure determination of 3.
2. Results
Although the original preparation of 1 involved simply heating catechol with thionyl chloride in benzene solution [], more recent reports have involved addition of pyridine and use of the highly toxic carbon disulfide as solvent [], and this was the method used in the preparation of 3 [], with the additional use of carbon tetrachloride. As we already reported for 1, simply heating catechol and thionyl chloride in toluene until the evolution of HCl ceased offered a much safer alternative [], and the application of the same method to the synthesis of 3 proved to be quite satisfactory. The reaction took an interesting course, however. Upon the addition of one equivalent of thionyl chloride to a toluene solution of 2-mercaptophenol (2-hydroxythiophenol) at room temperature, a vigorous exothermic reaction occurred with evolution of HCl, and a white solid was deposited. We interpret this as an attack of the more nucleophilic thiophenol function on thionyl chloride to give the dithiosulfite 5 (Scheme 2). Upon heating under reflux, the solid dissolved and a comproportionation reaction took place with the second equivalent of thionyl chloride to afford compound 3 in good yield.
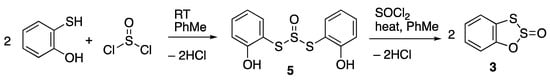
Scheme 2.
Improved synthesis of compound 3.
Although simple evaporation of the reaction solution gave product 3 in reasonable purity, it was not amenable to chromatographic purification owing to the ready hydrolysis on silica, and Kugelrohr distillation was accompanied by a significant degree of thermal decomposition with the evolution of sulfur dioxide and formation of an involatile polymeric resin. The distilled material was pure 3, obtained as a yellow liquid which crystallised upon cooling.
The 1H NMR spectrum of 3, described in the literature simply as δ 7.37 (m) [], showed an interesting and complex pattern (Figure 1). However, this was successfully simulated using the chemical shift values given in Table 1 and the coupling constants in Table 2 (Figure 1). The more symmetrical but still complex pattern in the 1H NMR spectrum of 1, described in the only literature report [] as δ 7.14 (AA′BB′), was likewise simulated (Figure 1) with values given in Table 1 and Table 2.
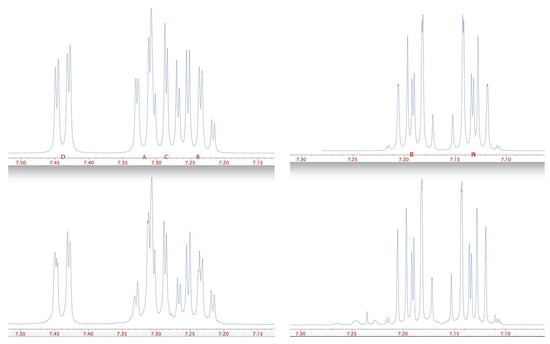

Table 1.
1H and 13C chemical shifts (ppm) and C–H coupling constants (Hz) for 3 and 1.

Table 2.
1H–1H coupling constants (Hz) in 3 and 1.
In order to achieve a full NMR assignment for compound 3, and for comparison 1, a range of additional spectra were obtained including DEPTQ 13C, HSQC, and HMBC (see Supplementary Materials). The HSQC data allowed the association of H and C shifts as shown in Table 1, but the HMBC results were key to arriving at an unambiguous assignment. Thus, for 3, the high carbon chemical shift of 153.9 ppm was clearly C–O, and this correlated on HMBC with the proton signal at 7.438 ppm, which was also correlated with the carbon at 127.2 ppm, while the carbon signal at 115.0 ppm was correlated on HMBC with the proton signal at 7.238. Similarly, for 1, the quaternary carbon signal at 142.7 ppm was correlated to the proton signal at 7.132 ppm which was also correlated to the carbon at 112.4, while the 124.4 carbon signal was linked by HMBC to the proton signal at 7.192. Combining this information with the coupling information derived from the proton spectra (Table 2) led to the unambiguous assignment of all the signals as summarised in Figure 2.
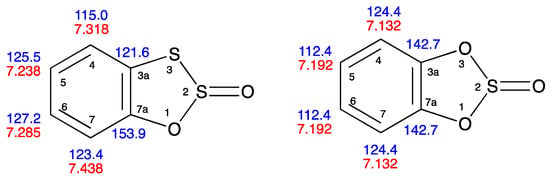
Figure 2.
Summary of 1H (red) and 13C (blue) chemical shift assignments for compounds 3 and 1.
Finally, in the NMR study, we obtained non-proton decoupled 13C NMR spectra of 3 and 1 from which the values of 1JC–H were readily determined, together with 2JC–H and even 3JC–H in some cases, and these values are also listed in Table 1. The values of 1JC–H, which have some relation to the state of hybridisation at the carbon atom involved, are consistent with those reported for other ortho-disubstituted benzene derivatives [].
Since compound 3 formed suitable crystals after distillation, we have been able to determine its structure by X-ray diffraction (Figure 3). The molecular structure has a planar benzene ring but the heterocyclic ring takes up an envelope conformation with the plane defined by O(1), S(2), and S(3) at an angle of 25.76(6)° to that defined by O(1), C(7a), C(3a), and S(3). Most surprisingly, the S=O oxygen adopts an “axial” position with the lone pair of S(2) in the equatorial position. As far as we are aware, this is the first structure of a 1,2,3-oxadithiole 2-oxide and indeed any 1,2,3-oxadithiole ring system.
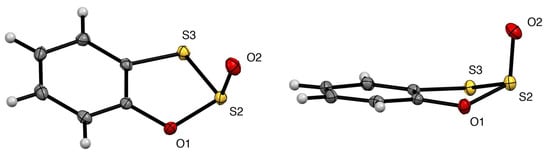
Figure 3.
Two alternative views of the molecular structure of 3 with probability ellipsoids at 50% level.
The structure of the oxygen analogue, catechol sulfite 1, has, however, been determined in the gas phase by electron diffraction [] and the structural parameters for the two compounds are compared in Table 3. Interestingly, the structure of 1 also features the “axial” S-oxide oxygen and a similar envelope conformation in the heterocyclic ring (Figure 4), although the flap angle in the case of 1 is significantly lower at 19.8(18)°. The other differences between 1 and 3 are rather predictable with a much lower internal ring angle and longer bonds to the ring S(3) as compared to ring O(3). The similar compound catechol sulfate 6 has been characterised twice by X-ray diffraction [,], and its structural parameters are also included in Table 3 for comparison. Again, in both structures, there is an envelope conformation with a lower flap angle of 14–15°.

Table 3.
Ring dimensions for 3, 1 [], and 6 [,] (Å, °).
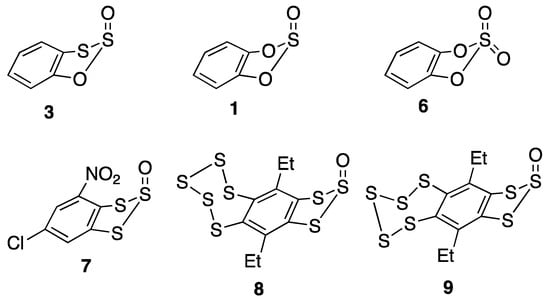
Figure 4.
Molecular structures of 3, 1, and 6–9 showing envelope conformation of the heterocyclic rings.
It is also interesting to note that the three crystallographically characterised 1,2,3-benzotrithiole 2-oxides, the chloro nitro compound 7 [], and the two isomeric pentathiepine compounds 8 and 9 [,] all show the same envelope conformation in the trithiole ring with oxygen axial.
In summary, we have been able to fully assign the 1H and 13C NMR spectra for compound 3, and for comparison 1, for the first time. The X-ray structure of 3, the first for a 1,2,3-oxadithiole, shows an envelope conformation of the heterocyclic ring with the 2-oxygen atom in an axial position, consistent with the structure of the oxygen analogue 1 previously determined by gas-phase electron diffraction.
3. Experimental
3.1. General Experimental Details
Melting points were recorded on a Reichert hot-stage microscope (Reichert, Vienna, Austria) and are uncorrected. NMR spectra were obtained using a Bruker AV300 instrument (Bruker, Billerica, MA, USA). Spectra were run with internal Me4Si as the reference and chemical shifts are reported in ppm to high frequency of the reference. NMR spectra were processed and simulations produced using iNMR reader, version 6.3.3 (Mestrelab Research, Santiago de Compostela, Spain). Compound 1 was prepared as previously described [].
3.2. Synthesis of Benzo[d][1,2,3]oxadithiole 2-Oxide 3
A solution of 2-mercaptophenol (5.0 g, 40 mmol) in toluene (50 mL) was stirred at RT, while thionyl chloride (3.0 mL, 4.89 g, 41 mmol) was added dropwise. After a few minutes, an exothermic reaction occurred with evolution of HCl gas and formation of a white solid. The mixture was heated under reflux for 3 h resulting in dissolution of the solid and further evolution of HCl. Evaporation gave the crude product (6.42 g) as a yellow liquid which was purified by Kugelrohr distillation (15 Torr, oven temp. 150–180 °C) to give the product (3.54 g, 51%) as a yellow liquid which crystallised on cooling to give 3 as yellow crystals, mp 44–46 °C (lit. [] 46.5 °C). For NMR data, see Figure 1 and Figure 2 and Table 1 and Table 2. IR data for compound 3 were already reported [].
3.3. X-ray Structure Determination of 3
X-ray diffraction data were collected at 100 K using a Rigaku FR-X Ultrahigh Brilliance Microfocus RA generator/confocal optics with XtaLAB P200 diffractometer [Mo Kα radiation (λ = 0.71073 Å), Tokyo, Japan]. Data were collected (using a calculated strategy) and processed (including correction for Lorentz, polarization, and absorption) using CrysAlisPro []. The structure was solved by dual-space methods (SHELXT) [] and refined by full-matrix least-squares against F2 (SHELXL-2019/3) []. Non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined using a riding model. All calculations were performed using the Olex2 interface [].
Crystal data for C6H4O2S2, M = 172.21 g mol−1, colourless prism, crystal dimensions 0.16 × 0.12 × 0.06 mm, monoclinic, space group P21/n (No. 14), a = 6.13102(15), b = 10.3500(3), c = 10.7854(3) Å, β = 100.979(2)°, V = 671.87(3) Å3, Z = 4, Dcalc = 1.702 g cm−3, T = 100 K, Goodness of fit on F2 1.082, 14199 reflections measured, 1661 unique (Rint = 0.0416), which were used in all calculations. The final R1 [I > 2σ(I)] was 0.0252 and wR2 (all data) was 0.0655. Data have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2340013. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures.
Supplementary Materials
The following supporting information is available online. cif and check-cif files for 3; 1H and 13C NMR data for 1 and 3.
Author Contributions
A.G. prepared the compound; D.B.C. and A.P.M. collected the X-ray data and solved the structure; R.A.A. designed the study, analysed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
A.G. thanks the Commissionerate of College Education, Government of Rajasthan for the generous support of a Foreign Training Program under its Teachers’ Interface for Excellence Scheme.
Data Availability Statement
The X-ray data are at CCDC as stated in the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anschütz, R.; Posth, W. Über zwei cyclische ester des brenzcatechins. Ber. Dtsch. Chem. Ges. 1894, 27, 2751–2753. [Google Scholar] [CrossRef]
- De Jongh, D.C.; Van Fossen, R.Y. Mass spectra and pyrolysis of o-phenylene sulfite and related compounds. J. Org. Chem. 1972, 37, 1129–1135. [Google Scholar] [CrossRef]
- Aitken, R.A.; Horsburgh, C.E.R. Flash vacuum pyrolysis of o-phenylene sulfite: Formation and purification of cyclopentadienone dimer. In Comprehensive Organic Chemistry Experiments for the Laboratory Classroom; Afonso, C.A.M., Candeias, N.R., Pereira Simão, D., Trinidade, A.F., Coelho, J.A.S., Tan, B., Franzén, R., Eds.; RSC: Cambridge, UK, 2016; Chapter 10.9; pp. 690–693. [Google Scholar] [CrossRef]
- Schulz, R.; Schweig, A. Cyclopentadienthion. Angew. Chem. Int. Ed. Engl. 1981, 20, 570–571. [Google Scholar] [CrossRef]
- Wilson, G.E., Jr.; Belkind, B.A. Teraoxysulfuranes from phenols. Synthesis and the dehydration of alcohols. J. Am. Chem. Soc. 1978, 100, 8124–8130. [Google Scholar] [CrossRef]
- Hansen, P.E. Carbon-hydrogen spin-spin coupling constants. Prog. NMR Spectrosc. 1981, 14, 175–295. [Google Scholar] [CrossRef]
- Schulz, G.; Serke, I.; Kapovits, I. Molecular structure of o-phenylene sulfite, an electron diffraction study. J. Chem. Soc. Faraday Trans. 2 1979, 75, 1612–1619. [Google Scholar] [CrossRef]
- Boer, F.P.; Flynn, J.J. Structural studies of strained cyclic esters. Catechol sulfate. J. Am. Chem. Soc. 1969, 91, 6604–6609. [Google Scholar] [CrossRef]
- Gembicky, M. Experimental Crystal Structure Determination. CCDC 1851728. 2018. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc204w57&sid=DataCite (accessed on 3 April 2024). [CrossRef]
- Kuzmich, A.S.; Khomenko, T.M.; Fedorov, S.N.; Makarieva, T.N.; Shubina, L.K.; Komarova, N.I.; Korchagina, D.V.; Rybalova, T.V.; Volcho, K.P.; Salakhutdinov, N.F. Cytotoxic and cancer preventive activity of benzotrithioles and benzotrithiole oxides, synthetic analogues of varacins. Med. Chem. Res. 2017, 26, 397–404. [Google Scholar] [CrossRef]
- Kimura, T.; Hanzawa, M.; Horn, E.; Kawai, Y.; Ogawa, S.; Sato, R. Preparation and conformational analysis of 6,10-disubstituted[1,2,3]trithiolo[h]benzopentathiepin monoxides. Tetrahedron Lett. 1997, 38, 1607–1610. [Google Scholar] [CrossRef]
- Kimura, T.; Hanzawa, M.; Tsujimura, K.; Takahashi, T.; Kawai, Y.; Horn, E.; Fujii, T.; Ogawa, S.; Sato, R. Preparation and conformational analysis of 6,10-diethyl[1,2,3]trithiolo[4,5-h]benzopentathiepin monoxides: Isolation and optical properties of chiral benzopentathiepin derivatives. Bull. Chem. Soc. Jpn. 2002, 75, 817–824. [Google Scholar] [CrossRef]
- CrysAlisPro v1. 171.42.96a Rigaku Oxford Diffraction; Rigaku Co.: Tokyo, Japan, 2023. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).