Unexpected Formation of the Iodobismuthate Salt (C14H15S2N2)2(C9H10SN)2[Bi4I16] upon Reaction of the Unsaturated Ligand Z-PySCH2CH=CHCH2SPy with BiI3
Abstract
:1. Introduction
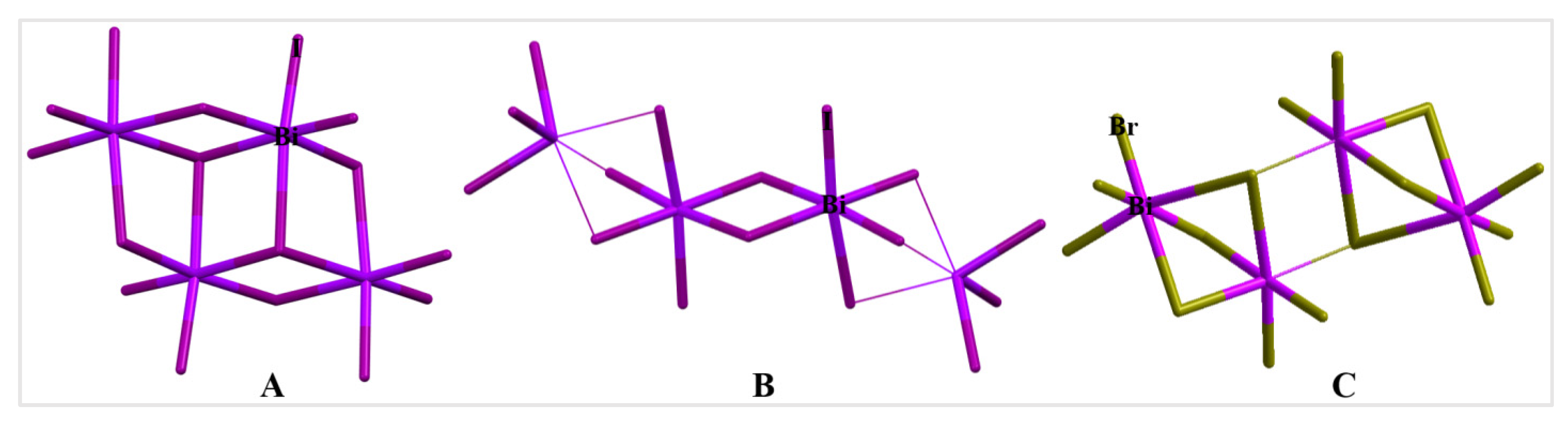
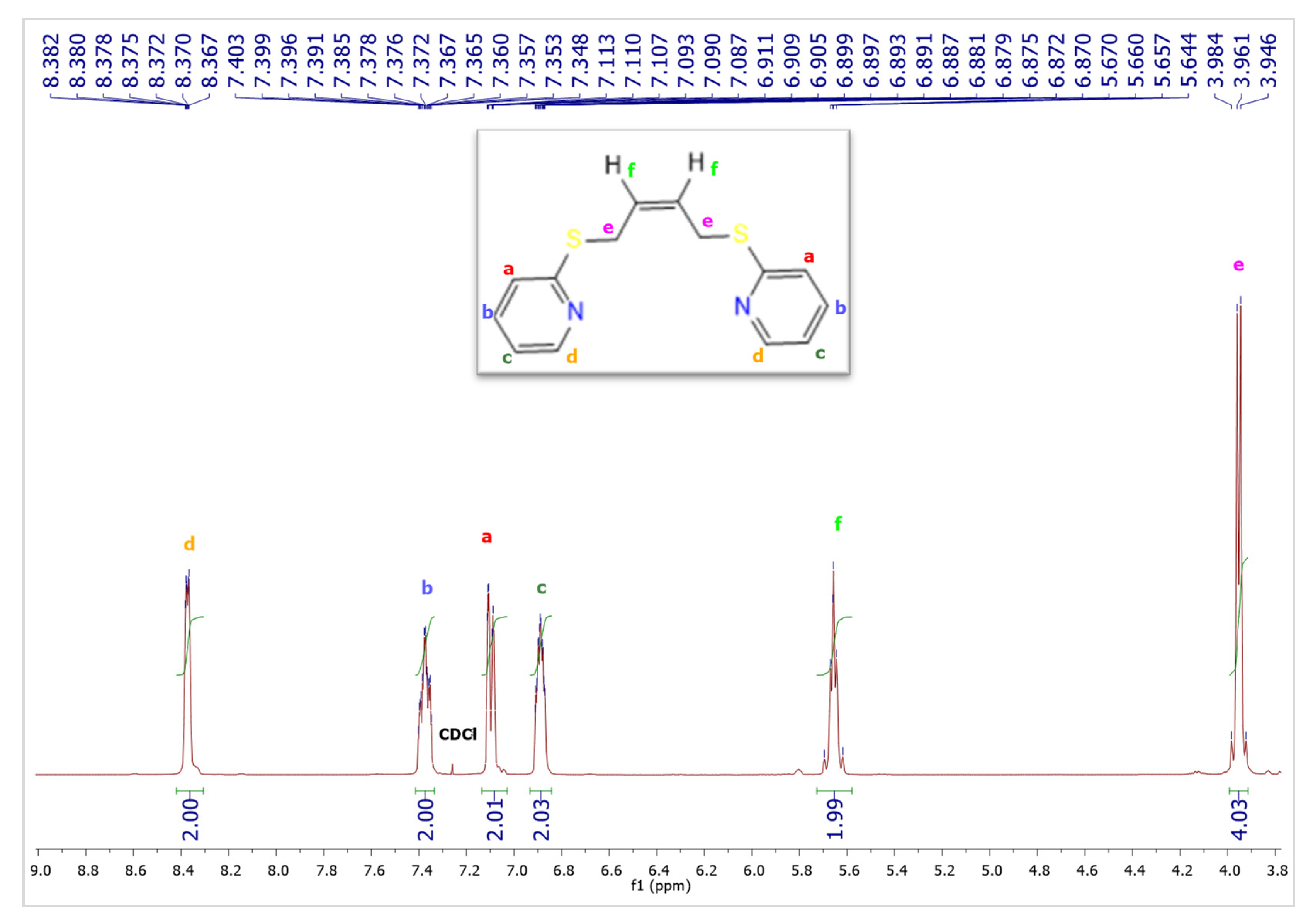
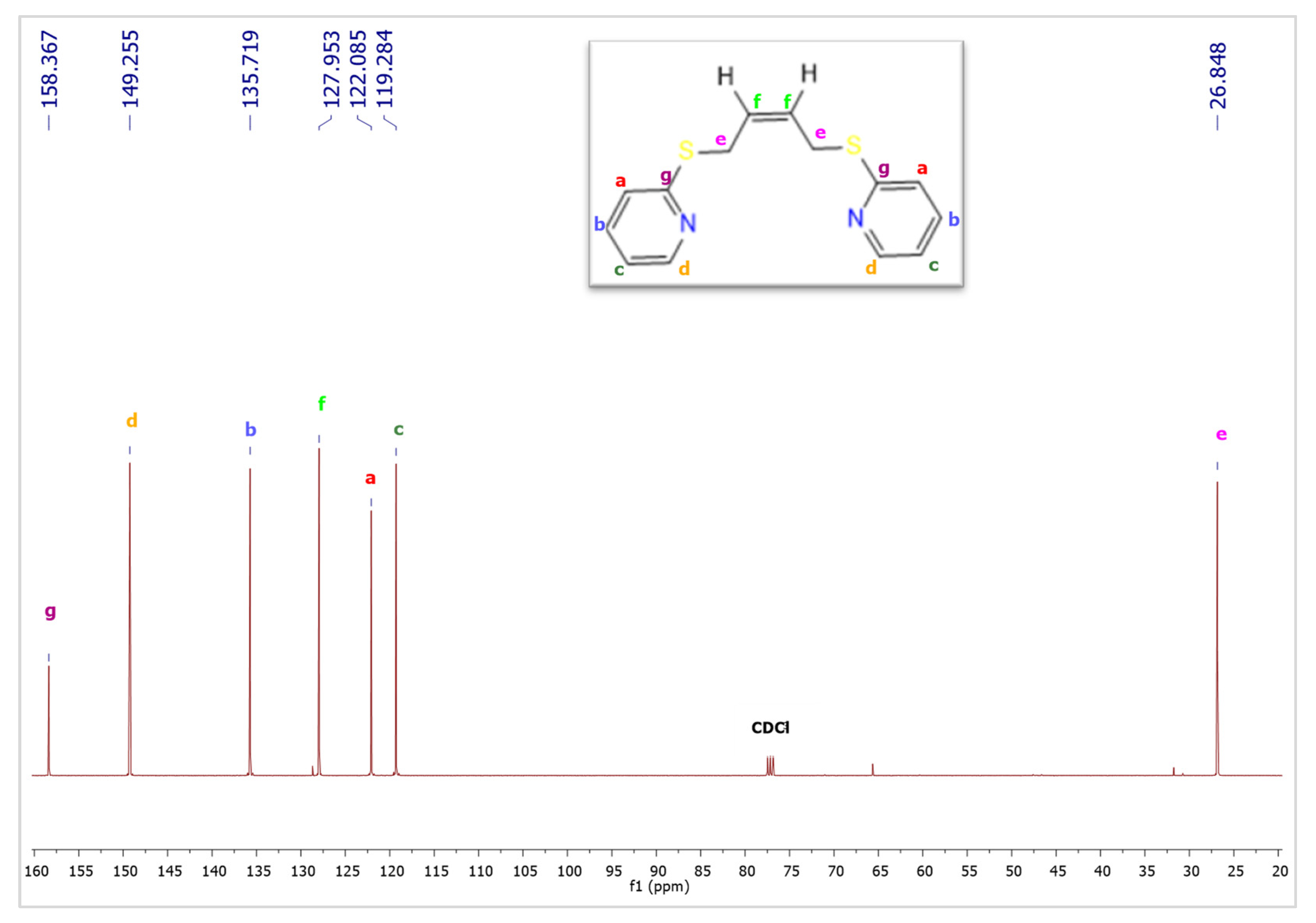
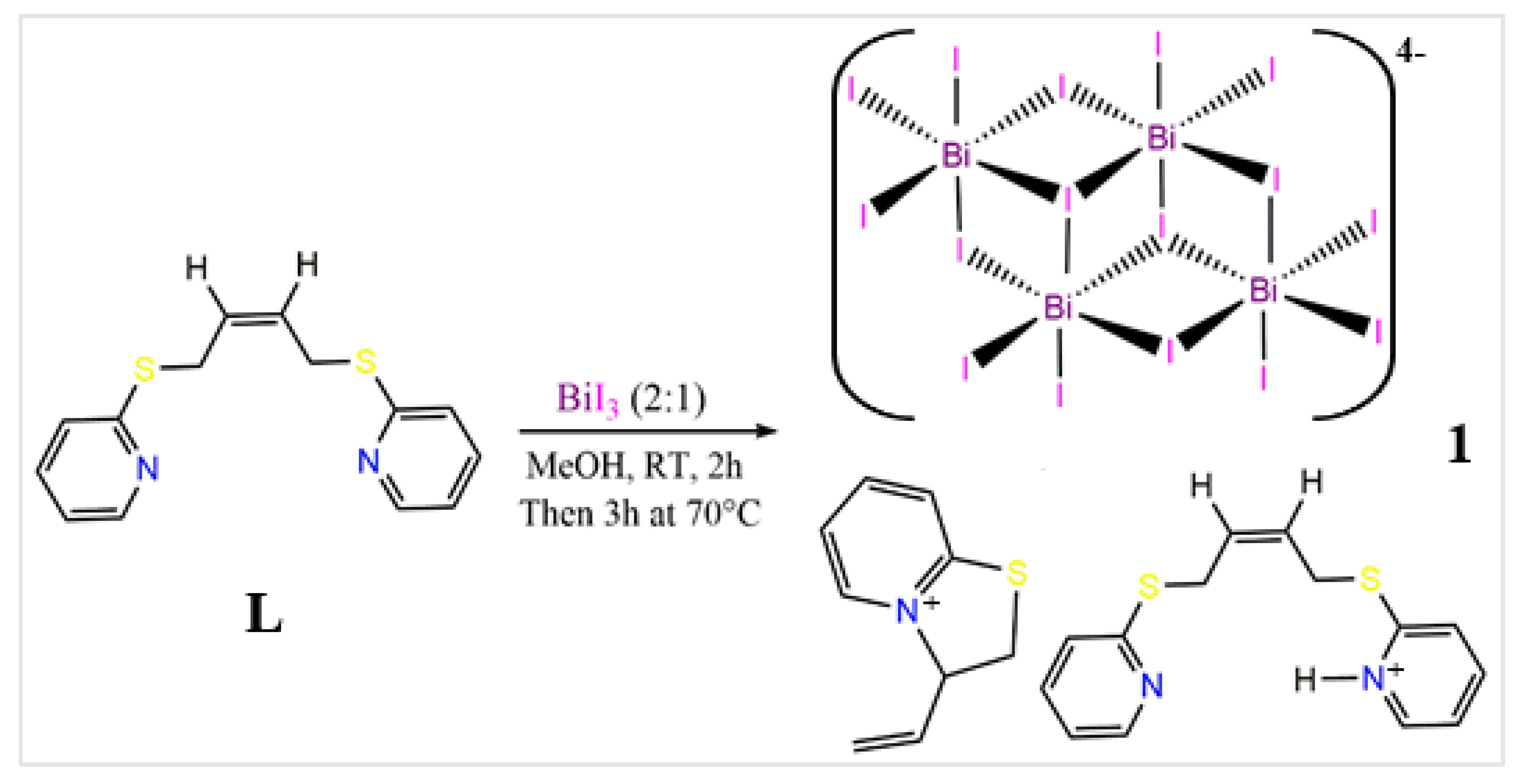
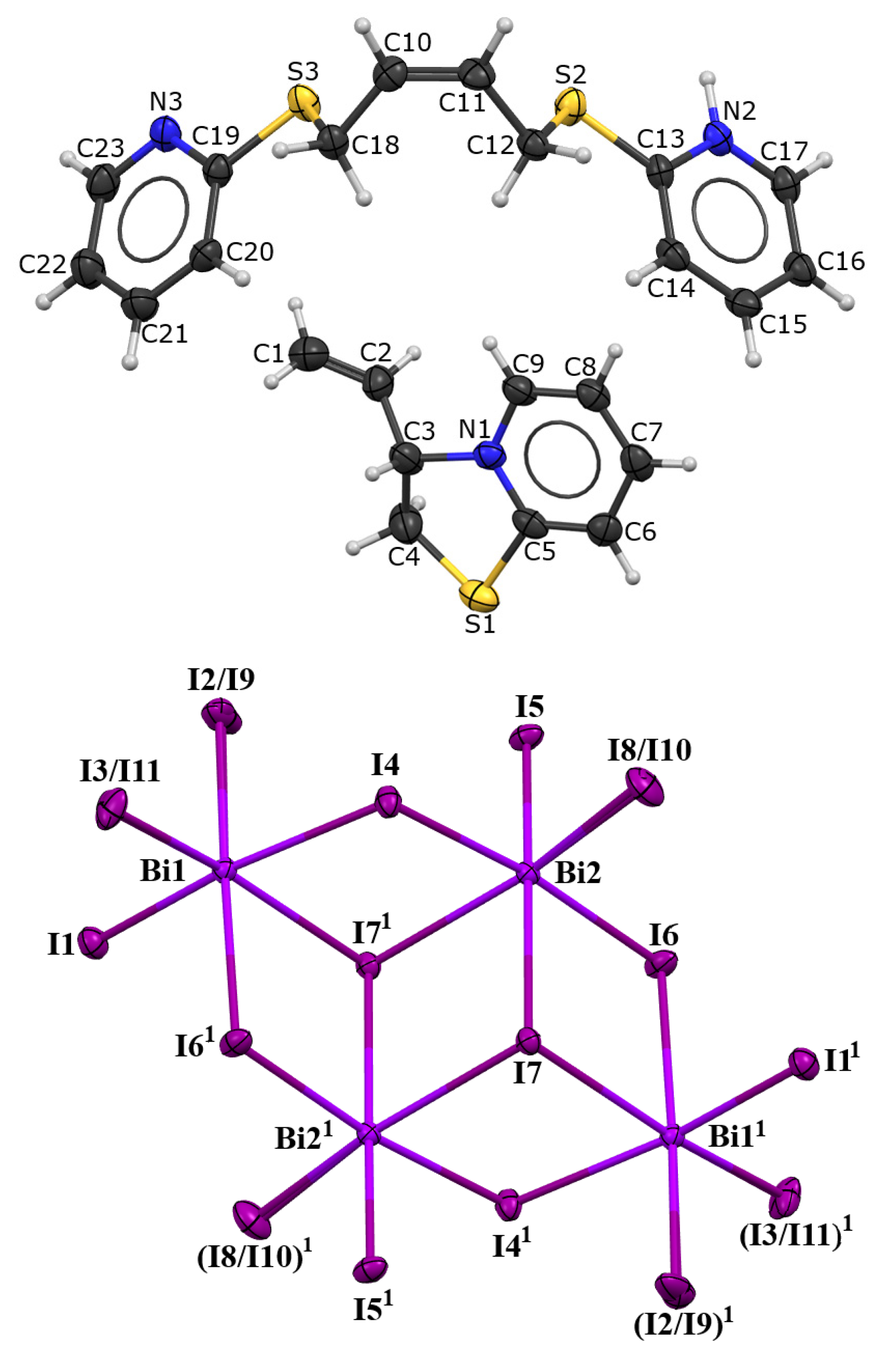
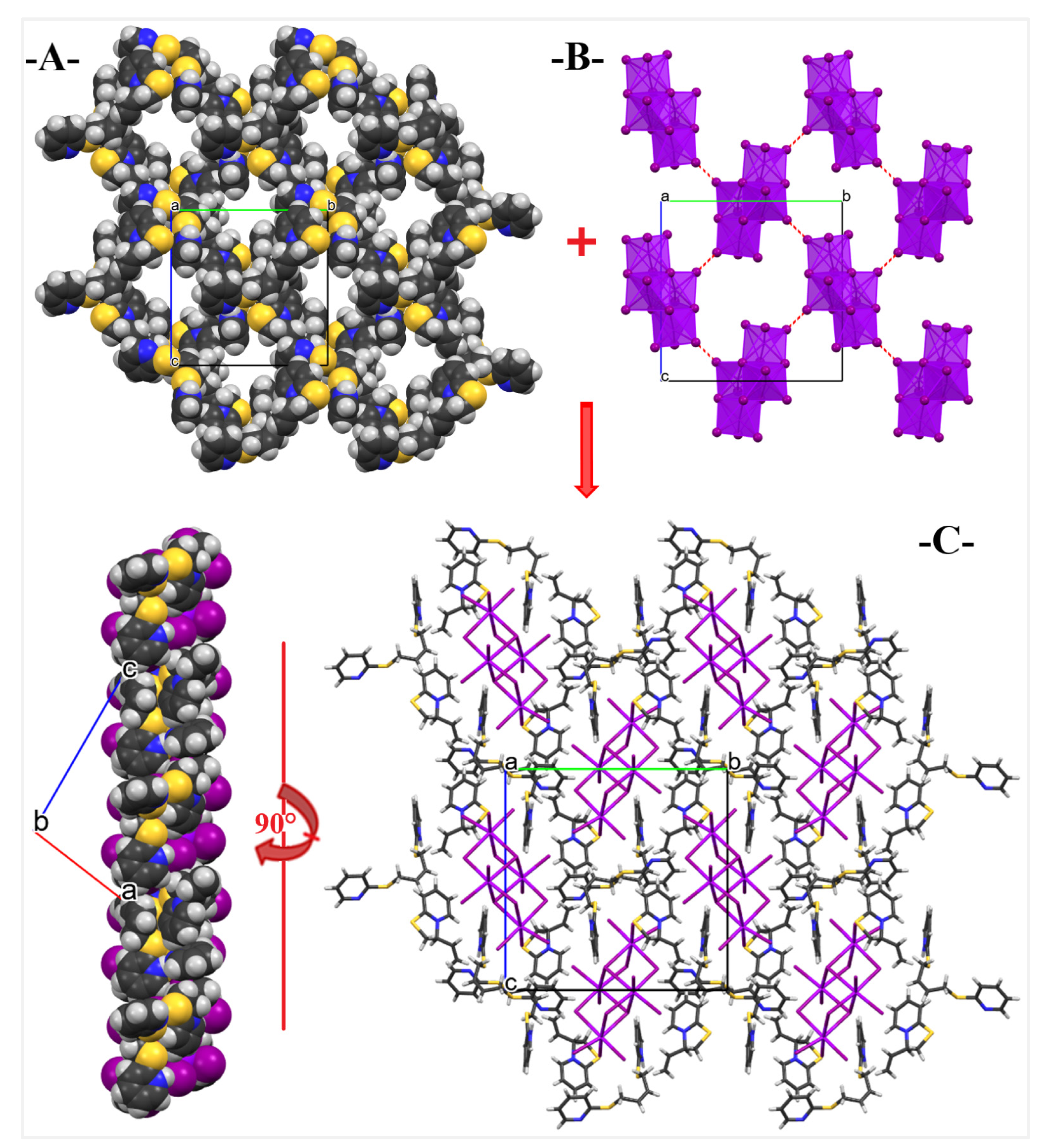
2. Results and Discussion
3. Discussion
4. Experimental
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Fernández, A.; Marcos-Cives, I.; Platas-Iglesias, C.; Castro-García, S.; Vázquez-García, D.; Fernández, A.; Sánchez-Andújar, M. Diimidazolium Halobismuthates [Dim]2[Bi2X10] (X = Cl−, Br−, or I−): A New Class of Thermochromic and Photoluminescent Materials. Inorg. Chem. 2018, 57, 7655–7664. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Wang, W.; Shen, J.; Fu, Y. Non-Transient Thermo-/Photochromism of Iodobismuthate Hybrids Directed by Solvated Metal Cations. Dalton Trans. 2020, 49, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Hrizi, C.; Samet, A.; Abid, Y.; Chaabouni, S.; Fliyou, M.; Koumina, A. Crystal structure, vibrational and optical properties of a new self-organized material containing iodide anions of bismuth (III), [C6H4(NH3)2]2Bi2I10·4H2O. J. Mol. Struct. 2011, 992, 96–101. [Google Scholar] [CrossRef]
- Hrizi, C.; Trigui, A.; Abid, Y.; Chniba-boudjada, N.; Bordet, P.; Chaabouni, S. α- to β-[C6H4(NH3)2]2Bi2I10 reversible solid-state transition, thermochromic and optical studies in the p-phenylenediamine-based iodobismuthate(III) material. J. Solid State Chem. 2011, 184, 3336–3344. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polynuclear Halide Complexes of Bi(III): From Structural Diversity to the New Properties. Coord. Chem. Rev. 2016, 312, 1–21. [Google Scholar] [CrossRef]
- Ouasri, A. Recent Advances on Structural, Thermal, Vibrational, Optical, Phase Transitions, and Catalysis Properties of Alkylenediammonium Halogenometallate Materials (Metal: Bi, Sb, Halogen: Cl, Br, I). Rev. Inorg. Chem. 2023, 43, 247–280. [Google Scholar] [CrossRef]
- Goforth, A.M.; Tershansy, M.A.; Smith, M.D.; Peterson, L., Jr.; Kelley, J.G.; DeBenedetti, W.J.I.; zur Loye, H.-C. Structural Diversity and Thermochromic Properties of Iodobismuthate Materials Containing D-Metal Coordination Cations: Observation of a High Symmetry [Bi3I11]2− Anion and of Isolated I− Anions. J. Am. Chem. Soc. 2011, 133, 603–612. [Google Scholar] [CrossRef]
- Ozório, M.S.; Dias, A.C.; Silveira, J.F.R.V.; Da Silva, J.L.F. Theoretical Investigation of the Role of Anion and Trivalent Cation Substitution in the Physical Properties of Lead-Free Zero-Dimensional Perovskites. J. Phys. Chem. C 2022, 126, 7245–7255. [Google Scholar] [CrossRef]
- Heine, J. A Step Closer to the Binary: The 1∞[Bi6I20]2− Anion. Dalton Trans. 2015, 44, 10069–10077. [Google Scholar] [CrossRef]
- Feldmann, C. Preparation and Crystal Structure of [Bi3I(C4H8O3H2)2(C4H8O3H)5]2Bi8I30 Containing the Novel Polynuclear [Bi8I30]6− Anion. J. Solid State Chem. 2003, 172, 53–58. [Google Scholar] [CrossRef]
- Hrizi, C.; Chaari, N.; Abid, Y.; Chniba-Boudjada, N.; Chaabouni, S. Structural Characterization, Vibrational and Optical Properties of a Novel One-Dimensional Organic–Inorganic Hybrid Based-Iodobismuthate(III) Material, [C10H7NH3]BiI4. Polyhedron 2012, 46, 41–46. [Google Scholar] [CrossRef]
- Hamdouni, M.; Hrizi, C.; Ahmed, H.E.; Knorr, M.; Krupp, A.; Strohmann, C.; Chaabouni, S. Reaction of Bi(NO3)3 with Quinoxaline in the Presence of HI. Synthesis of 5,6,7,8-Tetranitro-1,2,3,4-Tetrahydroquinoxaline-2,3-Diol by Serendipity: Crystal Structure, Hirshfeld and Optical Study of a Novel Energetic Compound. J. Mol. Struct. 2023, 1274, 134590. [Google Scholar] [CrossRef]
- Louvain, N.; Mercier, N.; Boucher, F. α- to β-(Dmes)BiI5 (Dmes = Dimethyl(2-Ethylammonium)Sulfonium Dication): Umbrella Reversal of Sulfonium in the Solid State and Short I···I Interchain Contacts—Crystal Structures, Optical Properties, and Theoretical Investigations of 1D Iodobismuthates. Inorg. Chem. 2009, 48, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Ferjani, H. Structural, Hirshfeld Surface Analysis, Morphological Approach, and Spectroscopic Study of New Hybrid Iodobismuthate Containing Tetranuclear 0D Cluster Bi4I16·4(C6H9N2)2(H2O). Crystals 2020, 10, 397. [Google Scholar] [CrossRef]
- Chen, W.-J.; Chu, K.-B.; Song, J.-L. Low-Dimensional Bismuth(III) Iodide Hybrid Material with High Activity for the Fast Removal of Rhodamine B. Acta Crystallogr. C 2018, 74, 1744–1749. [Google Scholar] [CrossRef]
- Pike, R.D.; Marshall, N.E.; Martucci, A.L. Alkylpyridinium Iodobismuthates(III). J. Chem. Crystallogr. 2022, 52, 161–173. [Google Scholar] [CrossRef]
- Mahjoor, P.; Latturner, S.E. Synthesis and Structural Characterization of [Bpyr]4[V4O4Cl12] and [Bpyr]4[Bi4Cl16] Grown in Ionic Liquid [Bpyr][AlCl4] (Bpyr = 1-Butylpyridinium). Cryst. Growth Des. 2009, 9, 1385–1389. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Uhler, A.D.; Landers, A.G. Synthesis, Crystal Structure and Molecular Geometry of [(.Eta.5-C5H5)2Fe]4[Bi4Br16], the Ferrocenium Salt of a “Cluster of Octahedra” Hexadecabromotetrabismuthate Counterion. Inorg. Chem. 1983, 22, 3255–3258. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Guo, C.-X.; Ni, C.-Y.; Ren, Z.-G.; Li, H.-X.; Lang, J.-P. Iodine-Induced Solvothermal Formation of Viologen Iodobismuthates. Eur. J. Inorg. Chem. 2010, 33, 5326–5333. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Samsonenko, D.G. Organic-Inorganic Hybrid Iodobismuthate, [Bi(L)4(H2O)]Bi3I12, Based on Tris(2-Pyridyl)Phosphine Oxide (L): Synthesis, Structure and Air-Oxidation into [Bi(L)4]2[Bi4I16(I3)2]. Inorg. Chem. Commun. 2018, 93, 47–51. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Egorova, I.V.; Levchuk, M.V.; Bukvetskii, B.V.; Popov, D.Y. Reaction of Tetraphenylantimony Bromide with O-Tolylbismuth Bis(2,5-Dimethylbenzenesulfonate). The Formation of Tetranuclear Anion [Bi4Br16]4−. Russ. J. Coord. Chem. 2002, 28, 613–617. [Google Scholar] [CrossRef]
- Genge, A.R.J.; Levason, W.; Reid, J. Bismuth(III) thioether chemistry: The synthesis and structure of [Bi4Cl12(MeSCH2 CH2CH2 SMe)4]n·nH2O, a highly unusual network involving Bi4Cl4 rings and bridging dithioether ligands. Chem. Commun. 1989, 19, 2159–2160. [Google Scholar] [CrossRef]
- Clegg, W.; Norman, N.C.; Pickett, N.L. Synthesis and structure of [SMe3]2[Bi2I8(SMe2)2]: A dimethylsulphide complex of bismuth(III). Polyhedron 1993, 12, 1251–1252. [Google Scholar] [CrossRef]
- Arar, W.; Khatyr, A.; Knorr, M.; Strohmann, C.; Schmidt, A. Bis(μ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth. Molbank 2022, 2022, M1381. [Google Scholar] [CrossRef]
- Bonnot, A.; Knorr, M.; Guyon, F.; Kubicki, M.M.; Rousselin, Y.; Strohmann, C.; Fortin, D.; Harvey, P.D. 1,4-Bis(arylthio)but-2-enes as Assembling Ligands for (Cu2X2)n (X = I, Br; n = 1, 2) Coordination Polymers: Aryl Substitution, Olefin Configuration, and Halide Effects on the Dimensionality, Cluster Size, and Luminescence Properties. Cryst. Growth Des. 2016, 16, 774–788. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.-R.; Du, M.; Zou, R.-Q.; Bu, X.-H. Novel Silver(I) Coordination Polymers with a Series of Bis(arylthio)ether Ligands Bearing a trans-2-Butene Backbone. Cryst. Growth Des. 2005, 5, 215–222. [Google Scholar] [CrossRef]
- Sorg, J.R.; Wehner, T.; Matthes, P.R.; Sure, R.; Grimme, S.; Heine, J.; Müller-Buschbaum, K. Bismuth as a versatile cation for luminescence in coordination polymers from BiX3 /4,4′-bipy: Understanding of photophysics by quantum chemical calculations and structural parallels to lanthanides. Dalton Trans. 2018, 475, 7669–7681. [Google Scholar] [CrossRef]
- Yan, X.-W.; Haji-Hasani, E.; Morsali, A. Syntheses and structural characterization of two new nanostructured Bi(III) supramolecular polymers via sonochemical method. Ultrasonics Sonochem. 2016, 315, 129–134. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Tershansy, M.A.; Goforth, A.M.; Gardinier, J.R.; Smith, M.D.; Peterson, L.; zur Loye, H.-C. Solvothermal Syntheses, High- and Low-Temperature Crystal Structures, and Thermochromic Behavior of [1,2-Diethyl-3,4,5-Trimethyl-Pyrazolium]4[Bi4I16] and [1,10-Phenanthrolinium][BiI4]·(H2O). Solid State Sci. 2007, 9, 410–420. [Google Scholar] [CrossRef]
- Goforth, A.M.; Peterson, L.; Smith, M.D.; zur Loye, H.-C. Syntheses and Crystal Structures of Several Novel Alkylammonium Iodobismuthate Materials Containing the 1,3-Bis-(4-Piperidinium)Propane Cation. J. Solid State Chem. 2005, 178, 3529–3540. [Google Scholar] [CrossRef]
- Buikin, P.A.; Rudenko, A.Y.; Ilyukhin, A.B.; Kotov, V.Y. Synthesis and Properties of Hybrid Halobismuthates of N-Acetonylpyridinium Derivatives. Russ. J. Inorg. Chem. 2021, 66, 482–489. [Google Scholar] [CrossRef]
- Bi, W.; Mercier, N. Reversible dynamic isomerism change in the solid state, from Bi4I16 clusters to BiI4 1D chains in L-cystine based hybrids: Templating effect of cations in iodobismuthate network formation. CrystEngComm 2008, 44, 5743–5745. [Google Scholar] [CrossRef]
- Du, H.; Wang, C.; Li, Y.; Zhang, W.; Xu, M.; Li, S.; Lu, Y.; Niu, Y.; Hou, H. A supramolecular metal-organic framework derived from bismuth iodide and 4,4′-bipyridinium derivative: Synthesis, structure and efficient adsorption of dyes. Micropor. Mesopor. Mater. 2015, 214, 136–142. [Google Scholar] [CrossRef]
- Dennington, A.J.; Weller, M.T. Synthesis, structure and optoelectronic properties of hybrid iodobismuthate & iodoantimonate semiconducting materials. Dalton Trans. 2018, 47, 3469–3484. [Google Scholar] [CrossRef]
- Adonin, S.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- and Two-Dimensional Iodine-Rich Iodobismuthate(III) Complexes: Structure, Optical Properties, and Features of Halogen Bonding in the Solid State. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef]
- Yin, W.-Y.; Weng, Y.-G.; Jiang, M.; Yu, S.-K.; Zhu, Q.-Y.; Dai, J. A Series of Tetrathiafulvalene Bismuth Chlorides: Effects of Oxidation States of Cations on Structures and Electric Properties. Inorg. Chem. 2020, 59, 5161–5169. [Google Scholar] [CrossRef]
- Ounalli, C.; Essid, M.; Bruno, G.; Santoro, A.; Abid, S.; Aloui, Z. Synthesis, Crystallographic Structure, DFT Computational Studies and Hirschfeld Surface Analysis of a New Tetranuclear Anionic Bromobismuthate(III): [C12H20N2]2Bi4Br16·2H2O. J. Mol. Struct. 2021, 1243, 130916. [Google Scholar] [CrossRef]
- Vasiliev, A.A.; Bykov, A.V.; Shestimerova, T.A.; Bykov, M.A.; Goncharenko, V.E.; Shevelkov, A.V. Supramolecular Structures of New Tetranuclear Hydroxypiperidine Iodoantimonates(III). Russ. Chem. Bull. 2023, 72, 641–650. [Google Scholar] [CrossRef]
- Mercier, N.; Louvain, N.; Bi, W. Structural diversity and retro-crystal engineering analysis of iodometalate hybrids. CrystEngComm 2009, 11, 720–734. [Google Scholar] [CrossRef]
- Bozorth, R.M. The crystal structure of cadmium iodide. J. Am. Chem. Soc. 1922, 44, 2232. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Van Bolhuis, F.; Koster, P.B.; Migchelsen, T. Refinement of the Crystal Structure of Iodine at 110° K. Acta Crystallogr. 1967, 23, 90–91. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. University of Western Australia. 2017. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 1 June 2022).
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Charmant, J.P.H.; Norman, N.C.; Orpen, A.G.; Starbuck, J. 4,4′-Bipyridyl adduct of an iodobismuthate anion linked by a 4,4′-bipyridinium cation. Acta Crystallogr. 2003, E59, m1000–m1001. [Google Scholar] [CrossRef]
- Potapova, V.A.; Ishigeev, R.S.; Shkurchenko, I.V.; Amosova, S.V. Assembling of Thiazolo [3,2-a]pyridinium Salts via the Reaction of 2-Pyridinesulfenyl Halides with Vinyl Ethyl Ether. Russ. J. Gen. Chem. 2019, 89, 2601–2603. [Google Scholar] [CrossRef]
- Otvos, S.B.; Szecsenyi, Z.; Ferenc Fulop, F. Bismuth(III)-Catalyzed Hydration of Terminal Alkynes: Sustainable Synthesis of Methyl Ketones in Batch and Flow. ACS Sustain. Chem. Eng. 2019, 7, 13286–13293. [Google Scholar] [CrossRef]
- Ollevier, T. (Ed.) Bismuth-Mediated Organic Reactions; Springer: Berlin/Heidelberg, Germany, 2012; pp. 69–142, 179–198. [Google Scholar]
- Salvador, J.A.R.; Pinto, R.M.A.; Silvestre, S.M. Recent advances of bismuth(III) salts in organic chemistry: Application to the synthesis of heterocycles of pharmaceutical interest. Curr. Org. Synth. 2009, 6, 426–470. [Google Scholar] [CrossRef]
- Ollevier, T. New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 2013, 11, 2740–2755. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essid, M.; Hrizi, C.; Ammar, S.; Khatyr, A.; Knorr, M.; Schmidt, A.; Strohmann, C. Unexpected Formation of the Iodobismuthate Salt (C14H15S2N2)2(C9H10SN)2[Bi4I16] upon Reaction of the Unsaturated Ligand Z-PySCH2CH=CHCH2SPy with BiI3. Molbank 2024, 2024, M1755. https://doi.org/10.3390/M1755
Essid M, Hrizi C, Ammar S, Khatyr A, Knorr M, Schmidt A, Strohmann C. Unexpected Formation of the Iodobismuthate Salt (C14H15S2N2)2(C9H10SN)2[Bi4I16] upon Reaction of the Unsaturated Ligand Z-PySCH2CH=CHCH2SPy with BiI3. Molbank. 2024; 2024(1):M1755. https://doi.org/10.3390/M1755
Chicago/Turabian StyleEssid, Marwa, Chakib Hrizi, Salah Ammar, Abderrahim Khatyr, Michael Knorr, Annika Schmidt, and Carsten Strohmann. 2024. "Unexpected Formation of the Iodobismuthate Salt (C14H15S2N2)2(C9H10SN)2[Bi4I16] upon Reaction of the Unsaturated Ligand Z-PySCH2CH=CHCH2SPy with BiI3" Molbank 2024, no. 1: M1755. https://doi.org/10.3390/M1755
APA StyleEssid, M., Hrizi, C., Ammar, S., Khatyr, A., Knorr, M., Schmidt, A., & Strohmann, C. (2024). Unexpected Formation of the Iodobismuthate Salt (C14H15S2N2)2(C9H10SN)2[Bi4I16] upon Reaction of the Unsaturated Ligand Z-PySCH2CH=CHCH2SPy with BiI3. Molbank, 2024(1), M1755. https://doi.org/10.3390/M1755






