Abstract
New imidazolidine-2,4,5-triones with norabietic, nordehydroabietic, and adamantane substituents were synthesized by reacting oxalyl chloride and the corresponding ureas, providing good yields. Bioisosteric replacement of the ureide group with a parabanic acid fragment made it possible to increase the solubility of compounds and conduct biological studies. The compounds inhibit the DNA repair enzyme tyrosyl-DNA phosphodiesterase 1 in submicromolar concentrations. Cytotoxic concentrations were also studied on the glioblastoma cell line SNB19.
1. Introduction
Despite the variety of existing methods for treating cancer, the most commonly used treatment regimen in clinical practice consists of chemotherapy and radiation therapy. The action of cytostatic agents and ionizing radiation is aimed at damaging the DNA structure, and the highly efficient functioning of the DNA repair systems in cancer cells prevents the achievement of the desired result. The selective inhibition of DNA repair enzymes can lead to both increasing the effectiveness of the main therapy and overcoming resistance to it.
An enzyme that can serve as a molecular target in antitumor therapy is tyrosyl-DNA phosphodiesterase 1 (TDP1) [1]. TDP1 is a eukaryotic DNA repair enzyme that specifically cleaves the covalent bond between the 3′-phosphate group of DNA and the tyrosine associated with a small peptide remaining after proteolysis/denaturation of Top1 (topoisomerase 1) [2]. TDP1 plays a key role in repairing DNA damage caused by clinically used anticancer drugs such as topotecan and irinotecan [3]. Thus, selective inhibition of this enzyme can significantly enhance their effect [4]. In addition, TDP1 has broad substrate specificity, allowing it to remove various other 3′ blocking lesions. This enzyme is capable of removing from the 3′ end of DNA deoxyribonucleosides, ribonucleosides and their analogues used as antitumor and antiviral drugs, including acyclovir, cytarabine and zidovudine [5], as well as hydrolyzing 3′-phosphoglycolate groups [6,7], which result from oxidative DNA damage or damage by radiomimetic drugs such as bleomycin [8]. The substrates of this enzyme include, among other things, 3′-phosphoglycolates and 3′-apurinic/apyrimidinic sites (AP sites) formed in DNA under the action of ionizing radiation [9]. In addition to the ability to remove covalent adducts of Top1 and DNA, TDP1 can hydrolyze AP sites in DNA (formed during the BER process) and repair 3-DNA–peptide/protein cross-links [10].
A promising approach to the development of new TDP1 inhibitors is to carry out chemical modifications of natural molecules. Multiple classes of effective inhibitors have been found among derivatives of various plant metabolites (Figure 1). Conjugates of monoterpenoids and 3-arylcoumarins 1 and thiazolidinediones with thiophene and terpene substituents 2 are active in the lower micromolar range. The leaders in inhibitory characteristics are derivatives of usnic acid 3 and 4, which are active in nanomolar concentrations [11].
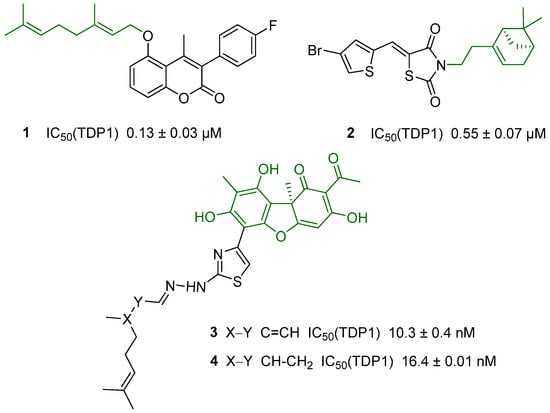
Figure 1.
TDP1 inhibitors containing a natural fragment. Natural fragment in the structure is indicated in green.
Previously, our team discovered a new class of effective inhibitors of the TDP1 repair enzyme—resin acid derivatives. Dehydroabietic and abietic acids are components of resins of coniferous plants; for example, a high acid content is found in the resin of Picea obovata [12]. Based on the diterpene compound dehydroabiethylamine, sets of ureas, thioureas, and biureas were synthesized and the resulting agents were shown to inhibit TDP1 in micromolar and submicromolar concentrations. For the first time, the synergistic effect of compound 5 with the alkylating agent temozolomide was studied on glioblastoma lines. The leading compound is able to improve its cytostatic properties by up to 40% (Figure 2) [13].
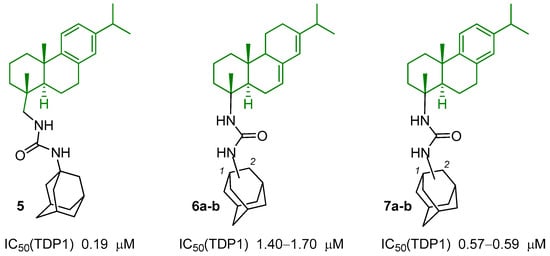
Figure 2.
Structures of TDP1 inhibitors based on resin acids and their derivatives. Resin acid fragment in the structure is indicated in green.
Attractive substance classes for the creation of new TDP1 inhibitors based on them are not only derivatives of dehydroabietic acid, but also other resin acids. Previously, our team obtained ureas with nordehydroabietic 6a–b and norabietic fragments 7a–b in the structure (Figure 2) [14]. The influence of the type of attachment of the adamantane fragment—in the first or second position of the scaffold—was studied. These compounds showed the ability to inhibit the DNA repair enzyme TDP1 in micromolar and submicromolar concentrations. However, further cellular experiments to study their cytostatic properties individually and in combinations could not be carried out due to their low solubility in water and organic solvents. To increase the solubility and bioavailability of certain classes of substances, a bioisosteric replacement of the ureide group with an imidazolidine-2,4,5-trione group can be used [15].
Imidazolidine-2,4,5-trione, or parabanic acid, is a natural metabolite and is formed in the body during the oxidation of uric acid. N-substituted derivatives of parabanic acid have found widespread use as inhibitors of important enzymes for the development of antitumor [16], antiepileptic [17], lipid-lowering [18] and antidiabetic therapies [19]. N-substituted derivatives of imidazolidine-2,4,5-triones have found use as effective inhibitors of soluble epoxide hydrolase (sEH) [20], acetylcholinesterase and butyrylcholinesterase [21]. Some trioxoimidazolidine compounds have demonstrated noticeable herbicidal properties [22]. Such a valuable heterocyclic structural block can be obtained on the basis of N-substituted ureas by reacting with oxalyl chloride [23,24] or diethyl oxalate under catalysis with dibutyltin oxide (DBTO) [25].
Thus, today, the creation of new tyrosyl-DNA phosphodiesterase inhibitors based on low-toxicity natural compounds is a promising approach to the development of multimodal approaches to anticancer therapy. Among these semisynthetic hybrid molecules, a significant number of compounds have been discovered that can enhance the effect of known chemotherapeutic drugs. The aim of the presented work was to synthesize new derivatives of abietic and dehydroabietic acids containing an adamantane fragment separated from the natural skeleton by an imidazolidine-2,4,5-trione linker and to study the biological properties of these agents.
2. Results
The starting substances for obtaining the target imidazolidine-2,4,5-triones were resin acids—abietic and dehydroabietic. According to the procedure described in the literature [14,26], unsymmetrical ureas with 1- and 2-adamantane fragments were synthesized. The imidazolidinetrione ring was obtained by reacting these ureas, 6a–b and 7a–b, with an excess of oxalyl chloride (Scheme 1). The reaction was carried out in methylene chloride with the addition of triethylamine for 1 h at room temperature. The target products were purified by means of column chromatography and isolated with good yields of 62–72%.
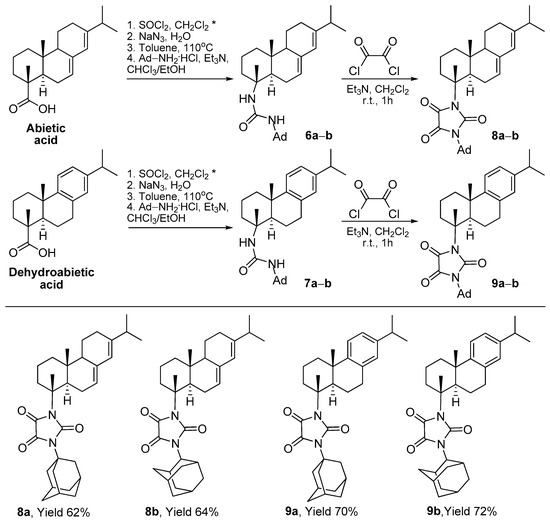
Scheme 1.
Synthesis of imidazolidine-2,4,5-triones. * Detailed synthetic procedures for compounds 6a–b and 7a–b have been described previously in [14].
It is expected that this transformation will increase the solubility of derivatives with nordehydroabietic and abietic fragments and expand the possibility for in vitro studies.
Primary screening of the obtained compounds was carried out against the TDP1 enzyme. The inhibitory properties of all synthesized compounds were studied using a technique previously developed at the Novosibirsk Institute of Chemical Biology and Fundamental Medicine [27]. For all derivatives obtained, IC50 values for the TDP1 enzyme were determined, presented in Table 1. Cytotoxic CC50 concentrations were determined using the standard MTT test [28] on the glioblastoma cell line SNB19.

Table 1.
Inhibitory activity of novel compounds against TDP1 and their cytotoxicity.
For compounds with a nordehydroabietic fragment 9a–b, replacing the urea group with an imidazolidinetrione group led to a slight decrease in the inhibitory characteristics against the TDP1 enzyme (IC50 = 0.78–0.96 µM for compounds 9a–b and IC50 = 0.57–0.59 µM for ureas 7a–b). Meanwhile, the cyclic norabietine derivatives 8a–b turned out to be two to three times more active (IC50 = 0.52–0.69 µM) than their ureide precursors 6a–b (IC50 = 1.40–1.70 µM). The solubility of all new compounds in DMSO and other organic solvents turned out to be significantly higher, which made it possible to study their cytotoxic properties on the glioblastoma cell line SNB19. For three compounds, the CC50 was above 100 µM, and only 9a showed noticeable toxicity. Thus, good inhibitory properties combined with low toxicity make this class of compounds interesting for further research. The introduction of parabanic acid fragments into target agents significantly increased the solubility of the substances, which is extremely important in the search for new effective biologically active compounds.
3. Materials and Methods
The 1H and 13C NMR spectra in CDCl3 were recorded on a Bruker AV-400 spectrometer (400.13 and 100.61 MHz, respectively). The residual signals of the solvent were used as references (δH 7.24, δC 76.90 for CDCl3). High-resolution mass spectra were recorded on a Thermo Scientific DFS instrument in full scan mode over the m/z range of 0–500 by means of ionization with an electron impact of 70 eV, and direct introduction of samples. IR spectra were recorded on a Vector22 spectrometer (KBr). Melting points were determined on a Termosystem FP 900 instrument from Mettler Toledo. Thin-layer chromatography was performed on Silufol plates (UV-254). The TLC plates were visualized via exposure to ultraviolet light (254 and 365 nm). Merck silica gel (63−200 μm) was used for column chromatography. The atomic numbering in the compounds was provided for the assignment of signals in the NMR spectra and was different from the atomic numbering in the systematic name. Copies of NMR, IR and high-resolution mass spectra are presented in the Supplementary Materials.
General procedure for the synthesis of imidazolidine-2,4,5-triones.
Compounds 6a–b and 7a–b (0.2 g, 0.00045 mol) were dissolved in 30 mL of CH2Cl2 with the addition of 0.4 mL of triethylamine. Oxalyl chloride (0.00225 mol, 0.285 g) was added dropwise while stirring with a magnetic stirrer. The conversion was monitored by TLC. Upon completion of the reaction (1 h), the reaction mixture was washed with distilled water (3 × 20 mL), and the organic extract was dried over Na2SO4. Then, the solvent was evaporated on a rotary evaporator. The final product was purified using silica gel column chromatography using hexane/ethyl acetate (9:1) as an eluent.
- 1-(1-Adamantyl)-3-[(1R,4aR,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthren-1-yl]imidazolidine-2,4,5-trione (8a) Yield 62%, white powder. M.p. 144.6 °C. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 5.74 (1H, s, H-14), 5.35 (1H, s, H-7), 0.98 and 0.97 (3H both, d, J16, 15 = 6.8, Me-16 and Me-17), 0.81 (3H, s, Me-18), 1.75 (3H, s, Me-19), 2.76–3.01 (1H, m, H-15), 2.28–2.50 (8H, m, 2H-24, 2H-25, 2H-26, H-5, H-6), 1.91–2.27 (7H, m, H-27, H-28, H-29, 2H-12, H-6, H-9), 1.50–1.87 (12H, m, 2H-30, 2H-31, 2H-32, 2H-2, H-11, H-1, 2H-3), 1.11–1.36 (2H, m, H-1, H-11). 13C NMR (100 MHz, CDCl3, δ, ppm): 157.43, 156.23 and 154.60 (C-20, C-21 and C-22), 145.63 (C-13), 135.53 (C-8), 122.10 (C-14), 119.80 (C-7), 29.59 (C-27, C-28, C-29), 35.81 (C-30, C-31, C-32), 39.92 (C-24, C-25, C-26), 20.83 and 20.70 (Me-17 and Me-16), 21.26 (Me-18), 13.85 (Me-19), 34.75 (C-15), 50.62 (C-9), 45.44 (C-5), 18.80 (C-2), 22.54 (C-11), 24.44 (C-6), 27.28 (C-12), 35.29 (C-10), 37.26 (C-1), 35.96 (C-3), 68.20 (C-4), 62.19 (C-23). IR (KBr), ν, cm−1: 3100–3600, 2915, 1726, 1356. Found, m/z: 504.3350 [M]+. (C32H44O3N2)+. Calculated, m/z: 504.3347.
- 1-(2-Adamantyl)-3-[(1R,4aR,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthren-1-yl]imidazolidine-2,4,5-trione (8b) Yield 64%, white powder. M.p. 111.4 °C. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 5.74 (1H, s, H-14), 5.34 (1H, s, H-7), 4.14 (1H, s br, H-23), 2.82–3.00 (1H, m, H-15), 0.98 and 0.97 (3H both, d, J16, 15 = 6.8, Me-16 and Me-17), 0.81 (3H, s, Me-19), 1.77 (3H, s, Me-18), 2.39–2.55 (3H, m, H-5, H-24, H-25), 1.11–1.33 (2H, m, H-1a, H-11a), 2.01–2.28 (6H, m, 2H-26, 2H-28, H-1, H-6), 1.71–2.01 (12H, m, 2H-27, 2H-29, H-30, H-31, 2H-32, H-9, H-6, 2H-12), 1.58–1.71 (5H, m, H-11, 2H-2, 2H-3). 13C NMR (100 MHz, CDCl3, δ, ppm): 157.77, 155.73 and 155.61 (C-20, C-21 and C-22), 145.66 (C-13), 135.58 (C-8), 122.02 (C-14), 119.65 (C-7), 20.71 (Me-17 and Me-16), 21.28 (Me-18), 13.87 (Me-19), 62.54 (C-23), 50.63 (C-9), 45.48 (C-5), 34.74 (C-15), 30.35 and 30.51 (C-24 and C-25), 27.17 and 26.45 (C-30 and C-31), 18.77 (C-2), 22.54 (C-11), 35.95 (C-3), 24.39 (C-6), 27.28 (C-12), 35.31 (C-10), 32.58 and 32.46 (C-26 and C-28), 68.28 (C-4), 37.25 (C-1), 37.89 and 37.94 (C-27 and C-29), 37.20 (C-32). IR (KBr), ν, cm−1: 3100–3600, 2916, 1726, 1339. Found, m/z: 504.3353 [M]+. (C32H44O3N2)+. Calculated, m/z: 504.3347.
- 1-(1-Adamantyl)-3-[(1R,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl]imidazolidine-2,4,5-trione (9a) Yield 70%, white powder. M.p. 96.8 °C. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 7.14 (1H, d, J = 8.1, H-11), 6.98 (1H, d, J = 8.1, H-12), 6.86 (1H, s, H-14), 1.74 (3H, s, Me-18), 1.20 (6H, d, J = 6.9, Me-16 and Me-17), 1.21 (3H, s, Me-19), 2.61–2.95 (5H, m, H-5, H-15, 2H-7, H-3), 2.37 (6H, br s, H-24, H-25, H-26), 2.19–2.30 (1H, m, H-1), 2.05–2.19 (3H, m, H-27, H-28, H-29), 1.62–1.71 (6H, m, 2H-30, 2H-31, 2H-32), 1.72–1.95 (4H, m, H-3, 2H-2, H-6), 1.46–1.59 (2H, m, H-1, H-6). 13C NMR (100 MHz, CDCl3, δ, ppm): 157.46, 156.24 and 154.62 (C-20, C-21 and C-22), 146.20 (C-9), 145.71 (C-13), 134.14 (C-8), 126.74 (C-14), 124.10 (C-11), 123.91 (C-12), 20.29 (Me-18), 23.83 and 23.86 (Me-17 and Me-16), 25.27 (Me-19), 33.31 (C-15), 44.60 (C-5), 29.54 (C-27, C-28, C-29), 19.13 (C-2), 19.93 (C-6), 29.66 (C-7), 35.77 (C-30, C-31, C-32), 39.89 (C-24, C-25, C-26), 69.24 (C-4), 38.44 (C-1), 36.80 and 35.10 (C-10 and C-3), 62.22 (C-23). IR (KBr), ν, cm−1: 2912, 1725, 1352. Found, m/z: 502.3187 [M]+. (C32H42O3N2)+. Calculated, m/z: 502.3190.
- 1-(2-Adamantyl)-3-[(1R,4aS,10aR)-7-isopropyl-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl]imidazolidine-2,4,5-trione (9b) Yield 72%, white powder. M.p. 89.3 °C. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 7.13 (1H, d, J = 8.2, H-11), 6.98 (1H, dd, J = 8.2, J = 1.7, H-12), 6.85 (1H, d, J = 1.7, H-14), 4.15 (1H, s br., H-23), 2.73–2.89 (4H, m, H-5, H-15, 2H-7), 2.65–2.73 (1H, m, H-3), 2.48–2.55 (2H, m, H-24, H-25), 2.21–2.29 (1H, m, H-1), 2.06–2.13 (2H, m, H-26, H-28), 1.71–1.98 (12H, m, 2H-27, 2H-29, H-30, H-31, 2H-32, H-3, 2H-2, H-6), 1.76 (3H, s, Me-18), 1.64–1.71 (2H, m, H-26, H-28), 1.48–1.60 (2H, m, H-1, H-6), 1.22 (3H, s, Me-19), 1.20 (6H, d, J = 6.9, Me-16 and Me-17). 13C NMR (100 MHz, CDCl3, δ, ppm): 157.68, 155.77 and 155.52 (C-20, C-21 and C-22), 146.08 (C-9), 145.67 (C-13), 134.03 (C-8), 126.69 (C-14), 124.00 (C-11), 123.86 (C-12), 69.25 (C-4), 62.55 (C-23), 44.61 (C-5), 38.38 (C-10), 37.77 (C-27 and C-29), 37.11 (C-32), 36.75 (C-1), 35.12 (C-3), 33.26 (C-15), 32.43 and 32.48 (C-26 and C-28), 30.25 and 30.27 (C-24 and C-25), 29.52 (C-7), 27.07 and 26.37 (C-30 and C-31), 25.19 (Me-19), 23.79 (Me-17 and Me-16), 20.14 (Me-18), 19.84 (C-6), 19.06 (C-2). IR (KBr), ν, cm−1: 2917, 1726, 1337. Found, m/z: 502.3188 [M]+. (C32H42O3N2)+. Calculated, m/z: 502.3190.
Supplementary Materials
Figures S1–S4: High resolution mass spectra of compounds 8a–9b; Figures S5–S8: 1H NMR and 13C NMR spectra of compounds 8a–9b; Figures S9–S12: IR spectra of compounds 8a–9b.
Author Contributions
Conceptualization, K.S.K. and O.I.Y.; methodology, K.S.K., O.I.Y., S.V.C. and A.L.Z.; investigation, K.S.K., A.A. and I.A.C.; resources, N.F.S., A.G.P. and O.I.L.; writing—original draft preparation K.S.K.; writing—review and editing, O.I.Y.; funding acquisition, K.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-23-10077, https://rscf.ru/project/23-23-10077/ accessed on 8 November 2023, and the Government of the Novosibirsk Region (No p-53).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Service Centre SB RAS for the spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baglini, E.; Salerno, S.; Barresi, E.; Robello, M.; Da Settimo, F.; Taliani, S.; Marini, A.M. Multiple Topoisomerase I (TopoI), Topoisomerase II (TopoII) and Tyrosyl-DNA Phosphodiesterase (TDP) inhibitors in the development of anticancer drugs. Eur. J. Pharm. Sci. 2021, 156, 105594. [Google Scholar] [CrossRef]
- Yang, S.W.; Burgin, A.B.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539. [Google Scholar] [CrossRef]
- Liang, X.; Wu, Q.; Luan, S.; Yin, Z.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur. J. Med. Chem. 2019, 171, 129–168. [Google Scholar] [CrossRef]
- Pommier, Y.; Huang, S.Y.N.; Gao, R.; Das, B.B.; Murai, J.; Marchand, C. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2). DNA Repair 2014, 19, 114–129. [Google Scholar] [CrossRef]
- Huang, S.Y.N.; Murai, J.; Dalla Rosa, I.; Dexheimer, T.S.; Naumova, A.; Gmeiner, W.H.; Pommier, Y. TDP1 repairs nuclear and mitochondrial DNA damage induced by chain-terminating anticancer and antiviral nucleoside analogs. Nucleic Acids Res. 2013, 41, 7793–7803. [Google Scholar] [CrossRef]
- Inamdar, K.V.; Pouliot, J.J.; Zhou, T.; Lees-Miller, S.P.; Rasouli-Nia, A.; Povirk, L.F. Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J. Biol. Chem. 2002, 277, 27162–27168. [Google Scholar] [CrossRef]
- Zhou, T.; Lee, J.W.; Tatavarthi, H.; Lupski, J.R.; Valerie, K.; Povirk, L.F. Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1). Nucleic Acids Res. 2005, 33, 289–297. [Google Scholar] [CrossRef]
- Hirano, R.; Interthal, H.; Huang, C.; Nakamura, T.; Deguchi, K.; Choi, K.; Bhattacharjee, M.B.; Arimura, K.; Umehara, F.; Izumo, S.; et al. Spinocerebellar ataxia with axonal neuropathy: Consequence of a Tdp1 recessive neomorphic mutation? EMBO J. 2007, 26, 4732–4743. [Google Scholar] [CrossRef]
- Katyal, S.; El-Khamisy, S.F.; Russell, H.R.; Li, Y.; Ju, L.; Caldecott, K.W.; McKinnon, P.J. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007, 26, 4720–4731. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Hinson, C.; Yang, K. 3-DNA—Peptide/protein cross-links arising from abasic sites in vitro. Nucleic Acids Res. 2022, 50, 1–20. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Chepanova, A.A.; Dyrkheeva, N.S.; Salakhutdinov, N.F.; Lavrik, O.I. Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1. Int. J. Mol. Sci. 2023, 24, 5781. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef]
- Kovaleva, K.; Oleshko, O.; Mamontova, E.; Yarovaya, O.; Zakharova, O.; Zakharenko, A.; Kononova, A.; Dyrkheeva, N.; Cheresiz, S.; Pokrovsky, A.; et al. Dehydroabietylamine Ureas and Thioureas as Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Enhance the Antitumor Effect of Temozolomide on Glioblastoma Cells. J. Nat. Prod. 2019, 82, 2443–2450. [Google Scholar] [CrossRef]
- Kovaleva, K.; Yarovaya, O.; Ponomarev, K.; Cheresiz, S.; Azimirad, A.; Chernyshova, I.; Zakharenko, A.; Konev, V.; Khlebnikova, T.; Mozhaytsev, E.; et al. Design, Synthesis, and Molecular Docking Study of New Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Inhibitors Combining Resin Acids and Adamantane Moieties. Pharmaceuticals 2021, 14, 422. [Google Scholar] [CrossRef]
- Burmistrov, V.; Morisseau, C.; Karlov, D.; Pitushkin, D.; Vernigora, A.; Rasskazova, E.; Butov, G.M.; Hammock, B.D. Bioisosteric substitution of adamantane with bicyclic lipophilic groups improves water solubility of human soluble epoxide hydrolase inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127430. [Google Scholar] [CrossRef]
- Sato, T.; Komine, T.; Masahiro, N.; Renbu, M.; Naoki, K. Novel Parabanic Acid Derivative and Drug Having the Same as Active Ingredient. WO2011078370A1, 30 June 2011. [Google Scholar]
- Aboutabl, M.E.; Hassan, R.M.; El-Azzouny, A.A.S.; Aboul-Enein, M.N.; Abd-Allah, W.H. Design and synthesis of novel parabanic acid derivatives as anticonvulsants. Bioorg. Chem. 2019, 94, 103473. [Google Scholar] [CrossRef]
- Ienaga, K.; Nakamura, K.; Ishii, A. Hypolipidemic Agent Containing Imidazolidin-Trione Derivative. JPH07165581A, 27 June 1995. [Google Scholar]
- Ishii, A.; Kotani, T.; Nagaki, Y.; Shibayama, Y.; Toyomaki, Y.; Okukado, N.; Ienaga, K.; Okamoto, K. Highly selective aldose reductase inhibitors. 1. 3-(Arylalkyl)-2,4,5-trioxoimidazolidine-1-acetic acids. J. Med. Chem. 1996, 39, 1924–1927. [Google Scholar] [CrossRef]
- Burmistrov, V.; Morisseau, C.; D’yachenko, V.; Karlov, D.; Butov, G.M.; Hammock, B.D. Imidazolidine-2,4,5- and pirimidine-2,4,6-triones—New primary pharmacophore for soluble epoxide hydrolase inhibitors with enhanced water solubility. Bioorganic Med. Chem. Lett. 2019, 30, 126908. [Google Scholar] [CrossRef]
- Pejchal, V.; Stepankova, S.; Padelkova, Z.; Imramovsky, A.; Jampilek, J. 1,3-substituted imidazolidine-2,4,5-triones: Synthesis and inhibition of cholinergic enzymes. Molecules 2011, 16, 7565–7582. [Google Scholar] [CrossRef]
- Li, B.; Man, Y.; Bai, L.; Ji, H.; Shi, X.; Cui, D. Solution-Phase Parallel Syntheses of Herbicidal 1-Phenyl-2,4,5- Imidazolidinetriones and 2-Thioxo-4,5-Imidazolidinediones. High Throughput Screen 2013, 16, 78–82. [Google Scholar] [CrossRef]
- Rajabi, M.; Mansell, D.; Freeman, S.; Bryce, R.A. Structure-activity relationship of 2,4,5-trioxoimidazolidines as inhibitors of thymidine phosphorylase. Eur. J. Med. Chem. 2011, 46, 1165–1171. [Google Scholar] [CrossRef]
- Yao, H.; Liu, F.; Chen, J.; Li, Y.; Cui, J.; Qiao, C. Antischistosomal activity of N,N′-arylurea analogs against Schistosoma japonicum. Bioorg. Med. Chem. Lett. 2016, 26, 1386–1390. [Google Scholar] [CrossRef]
- Kunde, L.B.; Kalyani, V.S.; Gupte, S.P. Dibutyltin oxide catalyzed aminolysis of oxalate to carbamate, oxamate and derivatives of imidazolidine trione. Appl. Organomet. Chem. 2010, 24, 402–407. [Google Scholar] [CrossRef]
- Huang, W.G.; Wang, H.S.; Huang, G.B.; Wu, Y.M.; Pan, Y.M. Enantioselective friedel-crafts alkylation of N-methylindoles with nitroalkenes catalyzed by chiral bifunctional abietic-acid-derived thiourea-Zn II complexes. Eur. J. Org. Chem. 2012, 2012, 5839–5843. [Google Scholar] [CrossRef]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Bioorg. Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).