Diethyl (5-Benzyl-2-(4-(N′-hydroxycarbamimidoyl)phenyl)-5-methyl-4,5-dihydrofuran-3-yl)phosphonate
Abstract
1. Introduction
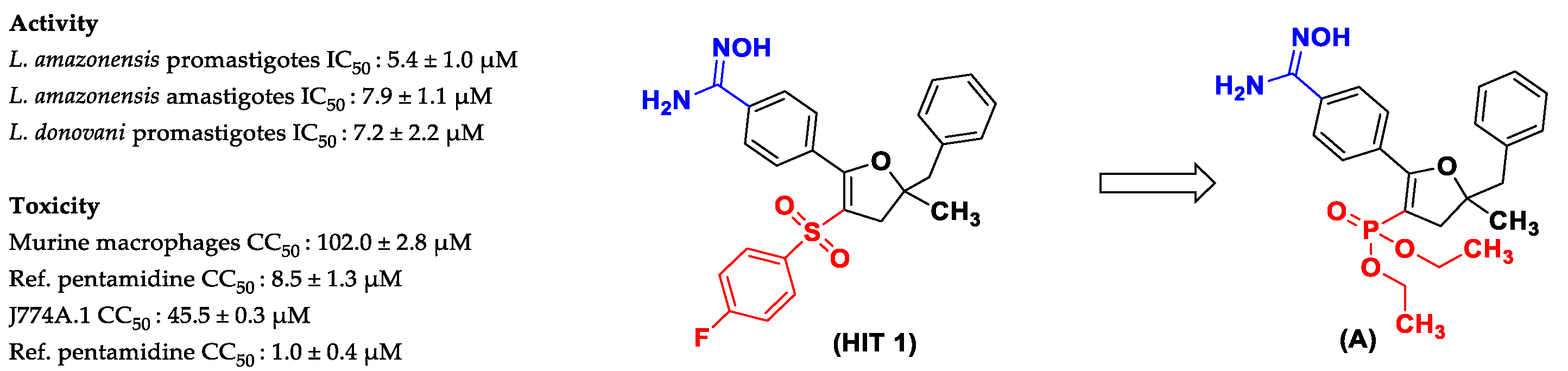
2. Results and Discussion
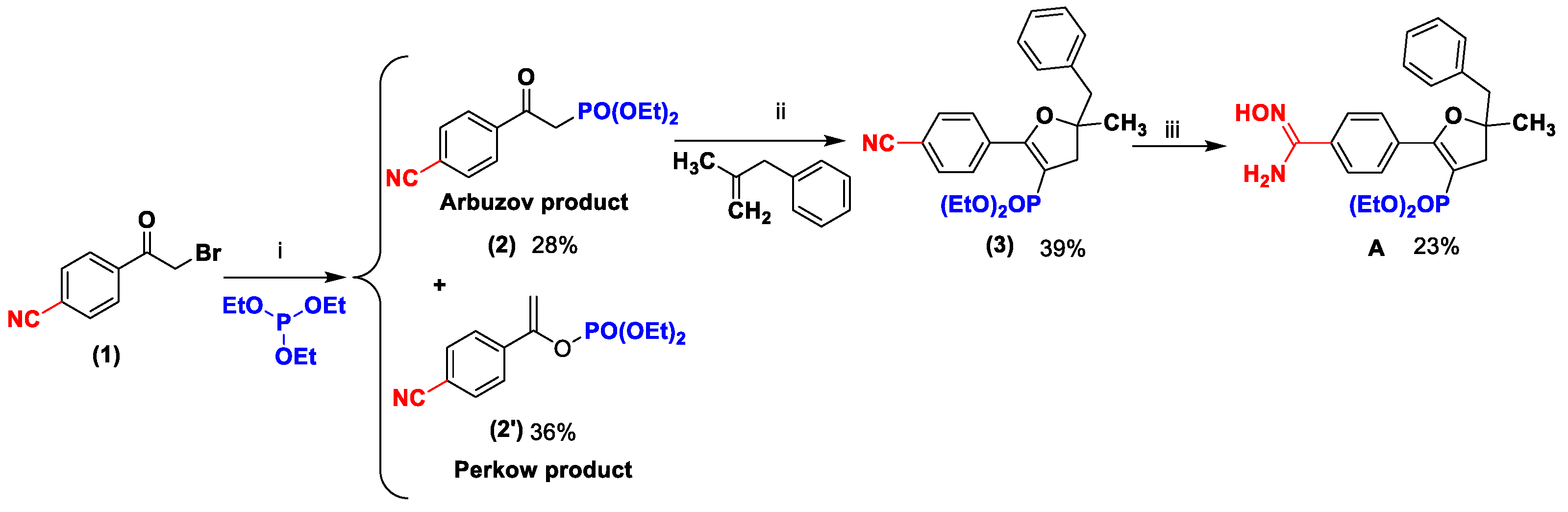
2.1. Synthesis
2.2. Biological Activity
3. Materials and Methods
3.1. Chemistry
3.2. Biology
3.2.1. Parasites
3.2.2. Antipromastigote Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization (WHO). World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals. Available online: www.who.int/data/gho/publications/world-health-statistics (accessed on 29 August 2023).
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Quintavalla, A.; Veronesi, R.; Carboni, D.; Martinelli, A.; Zaccheroni, N.; Mummolo, L.; Lombardo, M. Chemodivergent Photocatalytic Synthesis of Dihydrofurans and β,γ-Unsaturated Ketones. Adv. Synth. Catal. 2021, 363, 3267–3282. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, Z.; Jiang, X. Pd(0)-Catalyzed Coupling−Cyclization of 2-(2′,3′-Allenyl)Acetylacetates and Organic Halides: An Efficient Synthesis of 4,5-Dihydrofuran Derivatives. Org. Lett. 2007, 9, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Lin, B.; Gao, Y.; Ying, J.; Tang, G.; Zhao, Y. Mn(OAc)3-Mediated Synthesis of 3-Phosphonyldihydrofurans from β-Ketophosphonates and Alkenes. Synlett 2016, 28, 724–728. [Google Scholar] [CrossRef][Green Version]
- Curti, C.; Crozet, M.D.; Vanelle, P. Microwave-Assisted Manganese(III) Acetate Based Oxidative Cyclizations of Alkenes with β-Ketosulfones. Tetrahedron 2009, 65, 200–205. [Google Scholar] [CrossRef]
- Tabélé, C.; Faiões, V.D.S.; Grimaud, F.; Torres-Santos, E.C.; Khoumeri, O.; Curti, C.; Vanelle, P. Original Antileishmanial Hits: Variations around Amidoximes. Eur. J. Med. Chem. 2018, 148, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, H.; Shi, E.; Tang, W. Development and Clinical Application of Phosphorus-Containing Drugs. Med. Drug Discov. 2020, 8, 100063. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, I.J.; Firstenberg, S.; Borowitz, G.B.; Schuessler, D. Organophosphorus Chemistry. XVII. Kinetics and Mechanism of the Perkow Reaction. J. Am. Chem. Soc. 1972, 94, 1623–1628. [Google Scholar] [CrossRef]
- Afarinkia, K.; Cadogan, J.I.G.; Rees, C.W. Synthesis of 1,2-Azaphosphetidines. J. Chem. Soc. Chem. Commun. 1992, 3, 285. [Google Scholar] [CrossRef]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and Lead Criteria in Drug Discovery for Infectious Diseases of the Developing World. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, B.; Li, L.; Rao, K.; Yang, J.; Yu, F. Mn(OAc)s3–Promoted Oxidative Csp3–P Bond Formation through C sp2–C sp2 and P–H Bond Cleavage: Access to β-Ketophosphonates. J. Org. Chem. 2017, 82, 13268–13276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, M.; Zhou, Y.; Yang, K.; Su, J.; Du, J.; Song, Q. β-Ketophosphonate Formation via Aerobic Oxyphosphorylation of Alkynes or Alkynyl Carboxylic Acids with H-Phosphonates. Org. Lett. 2015, 17, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño Leon, O.L.; Curti, C.; Santos Urbancg Moncorvo, F.M.; Kabri, Y.; Redon, S.; Torres-Santos, E.C.; Paoli-Lombardo, R.; Vanelle, P. Diethyl (5-Benzyl-2-(4-(N′-hydroxycarbamimidoyl)phenyl)-5-methyl-4,5-dihydrofuran-3-yl)phosphonate. Molbank 2023, 2023, M1736. https://doi.org/10.3390/M1736
Avendaño Leon OL, Curti C, Santos Urbancg Moncorvo FM, Kabri Y, Redon S, Torres-Santos EC, Paoli-Lombardo R, Vanelle P. Diethyl (5-Benzyl-2-(4-(N′-hydroxycarbamimidoyl)phenyl)-5-methyl-4,5-dihydrofuran-3-yl)phosphonate. Molbank. 2023; 2023(4):M1736. https://doi.org/10.3390/M1736
Chicago/Turabian StyleAvendaño Leon, Oscar Leonardo, Christophe Curti, Fabiana Maia Santos Urbancg Moncorvo, Youssef Kabri, Sébastien Redon, Eduardo Caio Torres-Santos, Romain Paoli-Lombardo, and Patrice Vanelle. 2023. "Diethyl (5-Benzyl-2-(4-(N′-hydroxycarbamimidoyl)phenyl)-5-methyl-4,5-dihydrofuran-3-yl)phosphonate" Molbank 2023, no. 4: M1736. https://doi.org/10.3390/M1736
APA StyleAvendaño Leon, O. L., Curti, C., Santos Urbancg Moncorvo, F. M., Kabri, Y., Redon, S., Torres-Santos, E. C., Paoli-Lombardo, R., & Vanelle, P. (2023). Diethyl (5-Benzyl-2-(4-(N′-hydroxycarbamimidoyl)phenyl)-5-methyl-4,5-dihydrofuran-3-yl)phosphonate. Molbank, 2023(4), M1736. https://doi.org/10.3390/M1736






