Abstract
The synthesis of (E)-2-(1,3-diphenylallyl)-3,5-dimethoxyphenol is described by means of the reaction of 3,5-dimethoxyphenol with (E)-1,3-diphenylprop-2-en-1-ol in 1,1,1,3,3,3-hexafluoroispropanol (HFIP), which acts as a solvent and reaction promoter. The reaction proceeds smoothly to afford the mentioned compound in high yield under a metal and additive-free procedure. The corresponding allylated phenol has been fully characterized.
1. Introduction
The allylation reaction of electron-rich aromatics, such as phenol, in a Friedel–Crats-type reaction is a well-known transformation in organic synthesis [1,2,3]. However, this procedure normally requires the use of allylic substrates bearing a good leaving group, which are normally derived from alcohols, such as tosylates, carbonates, acetates, or halides. More recently, the use of alcohols as allylation substrates has also been achieved [4]. However, in both cases, the presence of a Brønsted or Lewis acid together with additives is frequently required in order to reach good yields (Scheme 1a). Thus, the overall process generates a stoichiometric amount of waste. Therefore, a much more attractive strategy from a practical, economical, and environmental point of view would be the direct use of alcohols without the need of such promoters and additives to carry out this transformation, generating water as the only by-product [5].
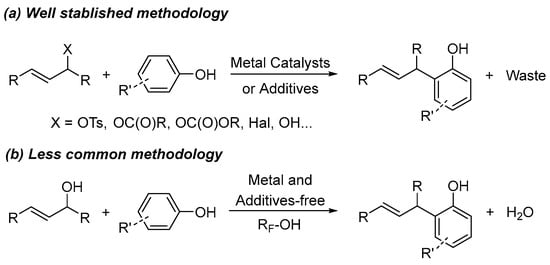
Scheme 1.
Allylation reaction of phenols.
With the aim of expanding the applicability of our ongoing project about the use of fluorinated alcohols as solvents and promoters of chemical transformation [6,7,8], we envisioned the use of such alcohols to carry out the above-mentioned transformation (Scheme 1b). This idea arose not only because of the unique chemical and physical properties (such as a high hydrogen bond donor ability, low nucleophilicity, high polarity and ionizing power values, and a slight Brønsted acidity) of fluorinated alcohols [9,10,11], but also because they have been shown to be able to promote the nucleophilic substitution reaction onto the so-called activated alcohols (such as benzylic and allylic alcohols) [12,13].
2. Results
The synthesis of phenol 3 was achieved following a well-established methodology developed within the group [12]. Thus, (E)-1,3-diphenylprop-2-en-1-ol (1) and 3,5-dimethoxyphenol (2) were allowed to react for 15 h at 50 °C using 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as a solvent and reaction promoter (Scheme 2). The solvent was then evaporated and, after purification by column chromatography, compound 3 was obtained in an 81% yield. The high regioselectivity of the reaction is also noteworthy, since the adduct 3 obtained from the attack of the para position of the OH in compound 2 was formally the sole observed (≥90%).
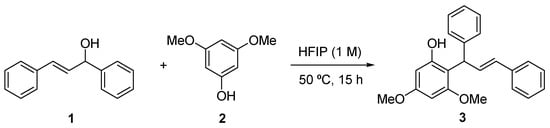
Scheme 2.
Synthesis of phenol 3.
It is important to remark that other polar molecules such as H2O or MeOH, which also have an important hydrogen bond donor ability, failed in this reaction, and no desired product 3 was observed. Contrarywise, when 2,2,2-trifluoroethanol (TFE) was employed, good conversion (81% by GC-MS) was also reached. However, in this case, a mixture of 5/1 between the formation of phenol 3 and ether 4 (Figure 1) was observed by analysis of GC-MS and NMR from the crude. The formation of this compound in this solvent could be ascribed by the lower acidity of this alcohol, which can act as a “base” in front of the phenol 2, hence allowing the formation of the nucleophilic phenolate. This situation would not occur in HFIP, where it is more acidic than phenol 2.
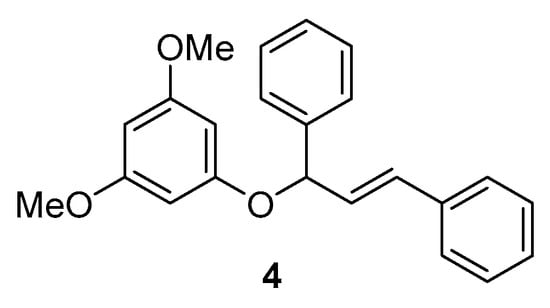
Figure 1.
Structure of ether 4.
Finally, an SN1-type process is proposed as a reaction mechanism (Scheme 3). Thus, firstly, an HFIP-mediated dehydration of allylic alcohol 1 would take place, rendering the quite stable delocalized allylic cation A. Then, the nucleophilic attack of 3,5-dimethoxyphenol (2) in a Friedel–Crafts type reaction onto this intermediate would take place. Finally, after rearomatization, the corresponding product 3 would be obtained.
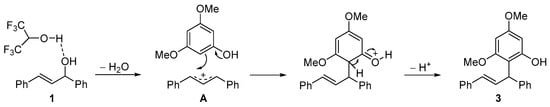
Scheme 3.
Proposed reaction mechanism.
3. Materials and Methods
All reagents and solvents were purchased from commercial suppliers and used without further purification. NMR spectra were performed on a Bruker AV-400 (Bruker Corporation, Karlsruhe, Germany) using CDCl3 as solvent. Low-resolution mass spectra (MS) were recorded in the electron impact mode (EI, 70 eV, He as carrier phase) using an Agilent GC/MS 5973 Network Mass Selective Detector spectrometer apparatus equipped with an HP-5MS column (Agilent Technologies, 30 m × 0.25 mm), with fragment ions given in m/z with relative intensities (%) in parentheses. High-resolution mass spectra (HRMS) were obtained on an Agilent 7200 Quadrupole–Time of Flight apparatus (Q-TOF) (Agilent Technologies, Palo Alto, CA, USA), with the ionization employed being electron impact (EI). IRs were recorded on a JASCO FT-IR 4100 LE Pike Miracle ATR (Jasco Inc., Jasco Analítica Spain, Madrid, Spain) and only the structurally most relevant peaks are listed. Analytical TLC was performed on Merck silica gel plates and the spots visualized with UV light at 254 nm (Merck Millipore, Billerica, MA, USA). Flash chromatography employed Merck silica gel 60 (0.040–0.063 mm).
General procedure for the HFIP-promoted synthesis of phenol 3.
In a capped tube, onto a mixture of (E)-1,3-diphenylprop-2-en-1-ol (1, 0.25 mmol) and 3,5-dimethoxyphenol (2, 0.5 mmol, 2 equiv.), HFIP (250 μL) was added in one portion. The reaction was then stirred at 50 °C for 15 h. After this time, solvent was evaporated, and the crude material was directly purified by flash chromatography.
(E)-2-(1,3-diphenylallyl)-3,5-dimethoxyphenol (3):
Slightly yellow sticky oil; purification by flash chromatography (hexane/EtOAc), 88% yield; Rf = 0.74 (hexane/ethyl acetate 3/2); IR (ATR): ν = 3247, 3027, 2962, 2840, 1601, 1494, 1455, 1205, 1147, 1097 cm−1; 1H NMR (400 MHz, CDCl3): δH = 7.47–7.41 (m, 2H), 7.38–7.30 (m, 6H), 7.29–7.21 (m, 2H), 6.87 (dd, J = 16.0, 6.9 Hz, 1H), 6.48 (dd, J = 16.0, 1.5 Hz, 1H), 6.19 (d, J = 2.4 Hz, 1H), 6.11 (d, J = 2.4 Hz, 1H), 5.57 (dd, J = 6.9, 1.2 Hz, 1H), 5.33 (s, 1H), 3.80 (s, 6H) ppm; 13C NMR (101 MHz, CDCl3): δC = 160.2, 158.7, 156.0, 142.1, 137.1, 132.1, 130.6, 128.7, 128.5, 127.9, 127.4, 126.6, 126.4, 109.5, 94.6, 91.9, 55.9, 55.3, 42.3 ppm; MS (EI): m/z 346 (M+, 100%), 347 (25), 329 (15), 315 (36), 255 (68), 241 (31), 192 (56), 167 (46), 91 (42); HRMS calcd for C23H22O3: 346,1569; found: 346.1559 (See Supplementary Materials).
4. Conclusions
In conclusion, we have herein described the synthesis of (E)-2-(1,3-diphenylallyl)-3,5-dimethoxyphenol (3) in a metal- and additive-free strategy by using 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as a solvent and promoter in the reaction between (E)-1,3-diphenylprop-2-en-1-ol (1) and 3,5-dimethoxyphenol (2). The corresponding product was obtained in high yield under smooth reaction conditions. In addition, the implemented process possesses a high atom economy, generating water as a by-product.
Supplementary Materials
The following materials are available online. 1H- NMR (Figure S1), 13C-NMR (Figure S2), GC-MS (Figure S3), and HRMS (Figure S4).
Author Contributions
Conceptualization, A.B.; methodology, L.M.-G. and A.B.; investigation, L.M.-G.; data curation, L.M.-G. and A.B.; writing—original draft preparation, L.M.-G. and A.B.; writing—review and editing, A.B.; supervision, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the Spanish Ministerio de Ciencia y Innovación (PID2021-127332NB-I00), Conselleria d’Innovació, Universitats, Ciència i Societat Digital de la Generalitat Valenciana (AICO/2021/013), and the University of Alicante (VIGROB-316) for the financial support. L.M.-G. would like to thank the University of Alicante for financial support (AII22-13).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vekariya, R.H.; Aubé, J. Hexafluoro-2-propanol-Promoted Intermolecular Friedel–Crafts Acylation Reaction. Org. Lett. 2016, 18, 3534–3537. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-J.; Milcent, T.; Crousse, B. Friedel–Crafts alkylation reaction with fluorinated alcohols as hydrogen-bond donors and solvents. RSC Adv. 2018, 8, 10314–10317. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mondal, S.; Tiwari, V.; Karmakar, T.; Kumar-Hazra, C. Cooperative Friedel–Crafts Alkylation of Electron-Deficient Arenes via Catalyst Activation with Hexafluoroisopropanol. Chem. Eur. J. 2023, 29, e2023001. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Nachtsheim, B.J. A review of new developments in the Friedel–Crafts alkylation—From green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Nájera, C. Recent Advances in the Direct Nucleophilic Substitution of Allylic Alcohols trough SN1-Type Reactions. Synthesis 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Llopis, N.; Baeza, A. HFIP-Promoted Synthesis of Substituted Tetrahydrofurans by Reaction of Epoxides with Electron-Rich Alkenes. Molecules 2020, 25, 3464–3476. [Google Scholar] [CrossRef]
- Llopis, N.; Baeza, A. Oxidation of Electron-Rich Arenes Using HFIP-UHP System. J. Org. Chem. 2020, 85, 6159–6164. [Google Scholar] [CrossRef] [PubMed]
- Llopis, N.; Gisbert, P.; Baeza, A. Direct Synthesis of N,N-Disubstituted Formamides by Oxidation of Imines Using an HFIP/UHP System. J. Org. Chem. 2020, 85, 11072–11079. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 88. [Google Scholar] [CrossRef]
- An, X.-D.; Xiao, J. Fluorinated Alcohols: Magic Reaction Medium and Promoters for Organic Synthesis. Chem. Rec. 2020, 20, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, H.F.; Armaly, A.M.; Cacioppo, J.G.; Coombs, T.C.; Koehn, K.R.K.; Norwood, V.M., IV; Aubé, J. HFIP in Organic Synthesis. Chem. Rev. 2022, 122, 12544–12747. [Google Scholar] [CrossRef] [PubMed]
- Trillo, P.; Baeza, A.; Nájera, C. Fluorinated Alcohols as Promoters for the Metal-Free Direct Substitution Reaction of Allylic Alcohols with Nitrogenated, Silylated, and Carbon Nucleophiles. J. Org. Chem. 2012, 77, 7344–7354. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Maquilón, C.; Ramón, D.J.; Baeza, A. Hexafluoroisopropanol-Promoted Metal-Free Allylation of Silyl Enol Ethers with Allylic Alcohols. Asian J. Org. Chem. 2017, 6, 1440–1444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).