Abstract
Sn(IV)-porphyrin complex with trans-dihydroxo axial-ligands and 2-pyridyl peripheral substituents, namely (trans-dihydroxo)[5,10,15,20-tetrakis(2-pyridyl)porphyrinato]tin(IV) was synthesized and fully characterized by various techniques such as elemental analysis, 1H NMR spectroscopy, ESI-MS spectrometry, UV-visible spectroscopy, and fluorescence spectroscopy.
1. Introduction
Sn(IV)-porphyrins are attractive motifs for constructing self-assembled metalloporphyrin nanostructures because they can exhibit unique features by adopting functional ligands in axial positions [1,2,3]. The role of hydroxo ligands in Sn(IV)-porphyrins has been investigated and exploited from various chemical perspectives. The X-ray structural analysis of (trans-dihydroxo)[5,10,15,20-tetrakis(4-pyridyl)porphyrinato]tin(IV) revealed that the hydroxo ligands act as a hydrogen bonding acceptor and the Sn(IV)-porphyrins are supramolecularly assembled into a two-dimensional network through hydrogen bonding [4]. The hydroxo ligand can be readily converted to alcoholic or carboxylic ligands by acidolysis, resulting in various hexacoordinate Sn(IV)-porphyrin complexes that exhibit interesting functions [5,6,7,8,9,10,11,12,13]. Sn(IV)-porphyrins with additional binding sites enable the creation of sophisticated multiporphyrin arrays [14,15,16] and nanostructures for application in photocatalysis [17,18,19]. Here, we report a tin(IV) porphyrin complex with trans-dihydroxo axial-ligand and 2-pyridyl peripheral substituents, namely, (trans-dihydroxo)[5,10,15,20-tetrakis(2-pyridyl)porphyrinato]tin(IV). Cooperativities between axial ligands and peripheral functional groups in six-coordinated Sn(IV)-porphyrins can lead to various assembled structures for the development of interesting porphyrin materials [20,21,22,23,24,25,26,27,28,29,30].
2. Results and Discussion
The synthetic route used for preparing free-base 5,10,15,20-tetrakis(2-pyridyl)porphyrin (H2T(2-Py)P) and its Sn(IV) complex (1) is shown in Scheme 1. In brief, H2T(2-Py)P was reacted with tin(II) chloride in pyridine under aerobic conditions followed by hydrolysis to afford 1 in high yield. 1 was fully characterized using various techniques, including elemental analysis, electrospray ionization mass (ESI-MS) spectrometry, 1H NMR, UV–vis absorption, and fluorescence spectroscopy. The 1H NMR spectrum of 1 in CDCl3 is shown in Figure S2. The resonance at −7.34 ppm of much higher field by the strong shielding of porphyrin ring current obviously represents the proton at the axial Sn-OH. On the other hand, the β-pyrrole and α-pyridyl protons of 1 are in the deshielding zone, and their resonances appear at 9.05−9.15 ppm. The remaining protons of the pyridyl group appear at 7.79, 8.17, and 8.35 ppm. 13C NMR confirmed the aromatic as well as porphyrinic carbon centers present at 1 as shown in Figure S3. In the FT-IR spectra of 1, the absorption peaks at 1025 cm−1 and 795 cm−1 belong to the bending vibration of C-H and the out-of-plane bending vibration of C-H in the aromatic ring, respectively. The peaks at 3050 cm−1, 1580 cm−1, and 1430 cm−1 are attributed to the stretching vibrations of C-H, C=C, and C-N in the pyrrole ring, respectively. The peaks at 3605 cm−1 are assigned to the stretching vibrations of the OH signal of the axial hydroxyl group present in 1. As shown in Figure S6, a peak at 771.12 is observed in the ESI-MS spectrum of 1, corresponding to the molecular ion peak [1 + H] +. The UV-vis absorption of 1 at 427 nm represents the strong Soret band, while the Q-bands appear at 522, 560, and 600 nm (Figure S7). This UV-vis absorption spectral pattern is quite similar to that of 4-pyridyl and 3-pyridyl analogues [5]. The fluorescence of 1 is observed as a two-band emission at 601 and 652 nm, as shown in Figure S8.
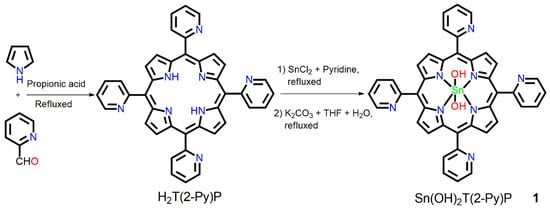
Scheme 1.
Synthesis of 1.
3. Materials and Methods
All chemicals were purchased from TCI (Tokyo Chemical Industry Co. LTD, Tokyo, Japan) and used without further purification. Elemental analysis was carried out using an EA 1110 Fisons analyzer (Used Lab Machines Limited, London, England). 1H NMR spectra were obtained using a Bruker BIOSPIN/AVANCE III 400 spectrometer at 293 K (Bruker BioSpin GmbH, Silberstreifen, Rheinstetten, Germany). Electrospray ionization mass (ESI-MS) spectra were recorded using a Thermo Finnigan linear ion trap quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Steady-state UV–Vis and fluorescence spectra were recorded using a Shimadzu UV-3600 and Shimadzu RF-5301PC fluorescence spectrophotometer, respectively (Shimadzu, Tokyo, Japan). FT-IR (Fourier transform infrared spectroscopy) spectra (KBr) were obtained using a Shimadzu FTIR-8400S spectrophotometer (Shimadzu, Tokyo, Japan).
3.1. 5,10,15,20-tetrakis(2-pyridyl)porphyrin H2T(2-Py)P
Freshly distilled pyrrole (1.10 mL, 16 mmol) was added dropwise to a solution of 2-pyridinecarboxaldehyde (1.52 mL, 16 mmol) in propionic acid (300 mL) under reflux. After 4 h, the solvent was evaporated to dryness under reduced pressure. Then, the resulting oily residue was dissolved in 10 mL of N,N’-dimethylformamide (DMF) and neutralized by adding 1 mL of triethylamine. Subsequently, the reaction mixture was kept in a refrigerator for 12 h. The solid precipitate was filtered, washed with hot water, and then dried in an oven. The crude compound was purified by column chromatography (SiO2, eluent: CHCl3/EtOH = 95:5) to afford 5,10,15,20-tetra(2-pyridyl)porphyrin H2T(2-Py)P. The product was recrystallized from CHCl3/n-hexane to give violet purple crystals. Yield: 0.544 g (22%). Anal. calculated for C40H26N8: C, 77.65; H, 4.24; N, 18.11. Found: C, 77.48; H, 4.57; N, 17.95. 1H NMR (400 MHz, CDCl3, ppm): δ −2.84 (s, 2H, NH), 7.71 (m, 4H, H4-Py), 7.89 (m, 4H, H3-Py), 8.21 (m, 4H, H5-Py), 8.85 (s, 8H, β-pyrrole), 9.14 (s, 4H, H2-Py). UV-vis (CHCl3): λnm (log ε), 419 (5.73), 516 (4.60), 552 (4.43), 593 (4.15), 650 (4.07). Emission (CHCl3): λnm 653, 712.
3.2. Synthesis of (trans-dihydroxo)[5,10,15,20-tetrakis(2-pyridyl)porphyrinato]tin(IV) (1)
H2T(2-Py)P (0.31 g, 0.5 mmol) was dissolved in 40 mL of pyridine, and SnCl2∙2H2O (0.225 g, 1 mmol) was added to the above solution and the mixture was refluxed for 12 h. After removal of all the volatiles in vacuo, the residue was dissolved in CHCl3 and filtered through a celite pad to remove the excess SnCl2. The filtrate was evaporated, and the solid residue was re-dissolved in a 3:1 mixture of THF and H2O (80 mL). K2CO3 (0.276 g, 2 mmol) was added to the reaction mixture, which was then refluxed for 12 h. After removal of THF under reduced pressure, the mixture was cooled to 10 °C until precipitation of a reddish compound. The filtered solid compound was dried in an oven. The crude product was recrystallized from CHCl3/CH3CN to give Sn(OH)2T(2-Py)P (1) as a red crystalline compound. Yield: 0.27 g (70%). Anal. calculated for C40H26N8O2Sn: C, 62.44; H, 3.41; N, 14.56; R, 19.59. Found: C, 62.18; H, 3.76; N, 14.43; R, 19.63. 1H NMR (400 MHz, CDCl3, ppm): δ −7.34 (s, 2H, Sn-OH), 7.79 (m, 4H, H4-Py), 8.17 (m, 4H, H3-Py), 8.38 (m, 4H, H5-Py), 9.05–9.15 (m, 12H, β-pyrrole + H2-Py). 13C NMR (400 MHz, CDCl3, ppm): δ 122.97, 123.02, 130,79, 132.68, 135.23, 146.60, 149.07, 159.31, 159.63. FT-IR (KBr): 3605, 3050, 1630, 1580, 1565, 1510, 1460, 1430, 1345, 1260, 1210, 1075, 1025, 990, 845, 795, 765, 725, 680, 555 cm−1. UV-vis (CHCl3): λnm (log ε), 427 (5.70), 522 (3.65), 560 (4.30), 600 (4.10). Emission (CHCl3): λnm 601, 652.
4. Conclusions
A tin(IV) porphyrin complex with trans-dihydroxo axial-ligands and 2-pyridyl peripheral substituents was synthesized and characterized by elemental analysis, 1H and 13C NMR spectroscopy, FT-IR spectroscopy, ESI-MS spectrometry, UV-visible spectroscopy, and fluorescence spectroscopy. This work can contribute to the design and synthesis of novel macrocyclic compounds for recognizing important biomolecules.
Supplementary Materials
Figures S1 and S2: 1H NMR spectra of free-base porphyrin and 1, Figure S3: 13C NMR spectrum of 1, Figure S4: FTIR spectrum of 1, Figures S5 and S6: ESI-MS spectra of free-base porphyrin and 1, Figure S7: UV-vis absorption spectra of free-base porphyrin and 1, Figure S8: Fluorescence spectra of free-base porphyrin and 1.
Author Contributions
Methodology, software, validation, formal analysis, investigation, data curation, visualization, writing—original draft preparation, N.K.S.; conceptualization, resources, writing—review and editing, project administration, funding acquisition, H.-J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Kumoh National Institute of Technology (2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We acknowledge Mihee Lee for the initial synthetic work.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Arnold, D.P.; Blok, J. The coordination chemistry of tin porphyrin complexes. Coord. Chem. Rev. 2004, 248, 299–319. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Hexacoordinated Sn(IV) porphyrin-based square-grid frameworks exhibiting selective uptake of CO2 over N2. Bull. Korean Chem. Soc. 2022, 43, 103–109. [Google Scholar] [CrossRef]
- Shee, N.K.; Jo, H.J.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Jo, H.-J.; Jung, S.-H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of trans-Dihydroxotin(IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]
- Fallon, G.D.; Langford, S.J.; Lee, M.A.-P.; Lygris, E. Self-assembling Mixed Porphyrin Trimers—The Use of Diaxial Sn(IV) Porphyrin Phenolates as an Organising Precept. Inorg. Chem. Commun. 2002, 5, 715–718. [Google Scholar] [CrossRef]
- Hawley, J.C.; Bampos, N.; Sanders, J.K.M. Synthesis and Characterization of Carboxylate Complexes of SnIV Porphyrin Monomers and Oligomers. Chem. Eur. J. 2003, 9, 5211–5222. [Google Scholar] [CrossRef]
- Ou, Z.; E, W.; Zhu, W.; Thordarson, P.; Sintic, P.J.; Crossley, M.J.; Kadish, K.M. Effect of Axial Ligands and Macrocyclic Structure on Redox Potentials and Electron-Transfer Mechanisms of Sn(IV) Porphyrins. Inorg. Chem. 2007, 46, 10840–10849. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Sn(IV) Porphyrin based axial-bonding type porphyrin triads containing heteroporphyrins as axial ligands. Inorg. Chem. 2010, 49, 2692–2700. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. A simple alternative method for preparing Sn(IV) porphyrins. J. Porphyrins Phthalocyanines 2010, 14, 361–370. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Supramolecular tetrads containing Sn(IV) porphyrin, Ru(II) porphyrin, and expanded porphyrins assembled using complementary metal–ligand interactions. Inorg. Chem. 2011, 50, 1713–1722. [Google Scholar] [CrossRef]
- Sharma, R.; Ghosh, A.; Wolfram, B.; Bröring, M.; Ravikanth, M. Synthesis and Characterization of Hexa-Coordinated Sn(IV) Complexes of Meso-Aryl Dipyrrins. Dalton Trans. 2013, 42, 5627–5630. [Google Scholar] [CrossRef]
- Pareek, Y.; Lakshmi, V.; Ravikanth, M. Axially bonded pentads constructed on the Sn(IV) porphyrin scaffold. Dalton Trans. 2014, 43, 6870–6879. [Google Scholar] [CrossRef]
- Natali, M.; Amati, A.; Demitri, N.; Iengo, E. Formation of a Long-Lived Radical Pair State in a Sn(IV) Porphyrin-di-(L-tyrosinato) Conjugate Driven by Proton-Coupled Electron-Transfer. Chem. Commun. 2018, 54, 6148–6152. [Google Scholar] [CrossRef]
- Shetti, V.S.; Pareek, Y.; Ravikanth, M. Sn(IV) Porphyrin Scaffold for Multiporphyrin Arrays. Coord. Chem. Rev. 2012, 256, 2816–2842. [Google Scholar] [CrossRef]
- Dvivedi, A.; Pareek, Y.; Ravikanth, M. SnIV Porphyrin Scaffolds for Axially Bonded Multiporphyrin Arrays: Synthesis and Structure Elucidation by NMR Studies. Chem. Eur. J. 2014, 20, 4481–4490. [Google Scholar] [CrossRef]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) Multiporphyrin Arrays as Tunable Photoactive Systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Morphology-controlled self-assembled nanostructures of complementary metalloporphyrin triads obtained through tuning their intermolecular coordination and their photocatalytic degradation of Orange II dye. Inorg. Chem. Front. 2022, 9, 5538–5548. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(iv)porphyrins with Re(i)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Inorg. Chem. Front. 2023, 10, 174–183. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Chong, C.; Forsyth, C.; Langford, S.J.; Woodward, C.P. Investigations of rotamers in diaxial Sn(IV)porphyrin phenolates—Towards a molecular timepiece. Tetrahedron 2008, 64, 8394–8401. [Google Scholar] [CrossRef]
- Lazarides, T.; Kuhri, S.; Charalambidis, G.; Panda, M.K.; Guldi, D.M.; Coutsolelos, A.G. Electron vs Energy Transfer in Arrays Featuring Two Bodipy Chromophores Axially Bound to a Sn(IV) Porphyrin via a Phenolate or Benzoate Bridge. Inorg. Chem. 2012, 51, 4193–4204. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.M.; Geisel, K.; Fetzer, A.; Kurz, P. A Water-Soluble Tin(IV) Porphyrin as a Bioinspired Photosensitiser for Light-Driven Proton-Reduction. Phys. Chem. Chem. Phys. 2014, 16, 12029–12042. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, K.; Yamazaki, T.; Suzuri, Y.; Nabetani, Y.; Onuki, S.; Takagi, S.; Shimada, T.; Tachibana, H.; Inoue, H. Hydrogen evolution coupled with photochemical oxygenation of cyclohexene with water sensitized by Tin (IV) porphyrins by visible light. Photochem. Photobiol. Sci. 2014, 13, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Alka, A.; Pareek, Y.; Shetti, V.S.; Rajeswara Rao, M.; Theophall, G.G.; Lee, W.-Z.; Lakshmi, K.V.; Ravikanth, M.V. Construction of Novel Cyclic Tetrads by Axial Coordination of Thiaporphyrins to Tin(IV) Porphyrin. Inorg. Chem. 2017, 56, 13913–13929. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, M.; Raghav, D.; Rathinasamy, K.; Kathiravan, A.; Mothi, E.M. DNA Targeting Long-Chain Alkoxy Appended Tin(IV) Porphyrin Scaffolds: Photophysical and Antimicrobial PDT Investigations. ACS Appl. Bio Mater. 2018, 1, 1705–1716. [Google Scholar] [CrossRef]
- Ravikumar, M.; Kathiravan, A.; Neels, A.; Mothi, E.M. Tin(IV) Porphyrins Containing β-Substituted Bromines: Synthesis, Conformations, Electrochemistry and Photophysical Evaluation. Eur. J. Inorg. Chem. 2018, 34, 3868–3877. [Google Scholar] [CrossRef]
- Baral, E.R.; Kim, D.; Lee, S.; Park, M.H.; Kim, J.G. Tin(IV)-Porphyrin Tetracarbonyl Cobaltate: An Efficient Catalyst for the Carbonylation of Epoxides. Catalysts. 2019, 9, 311. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kim, H.; Mergu, N.; Son, Y.-A. A photocatalytic comparison study between tin complex and carboxylic acid derivatives of porphyrin/TiO2 composites. Res. Chem. Intermed. 2020, 46, 313–328. [Google Scholar] [CrossRef]
- Giannoudis, E.; Benazzi, E.; Karlsson, J.; Copley, G.; Panagiotakis, S.; Landrou, G.; Angaridis, P.; Nikolaou, V.; Matthaiaki, C.; Charalambidis, G.; et al. Photosensitizers for H2 Based on Charged or Neutral Zn and Sn Porphyrins. Inorg. Chem. 2020, 59, 1611–1621. [Google Scholar] [CrossRef]
- Thomas, A.; Ohsaki, Y.; Nakazato, R.; Kuttassery, F.; Mathew, S.; Remello, S.N.; Tachibana, H.; Inoue, H. Molecular Characteristics of Water-Insoluble Tin-Porphyrins for Designing the One-Photon-Induced Two-Electron Oxidation of Water in Artificial Photosynthesis. Molecules 2023, 28, 1882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).