Synthesis of Catena-bis(μ-bromo)-(O-methyl-N-phenylthiocarbamate)-dicopper(I) and Its Reactivity towards PAr3 (Ar = Ph, p-Tol)
Abstract
1. Introduction
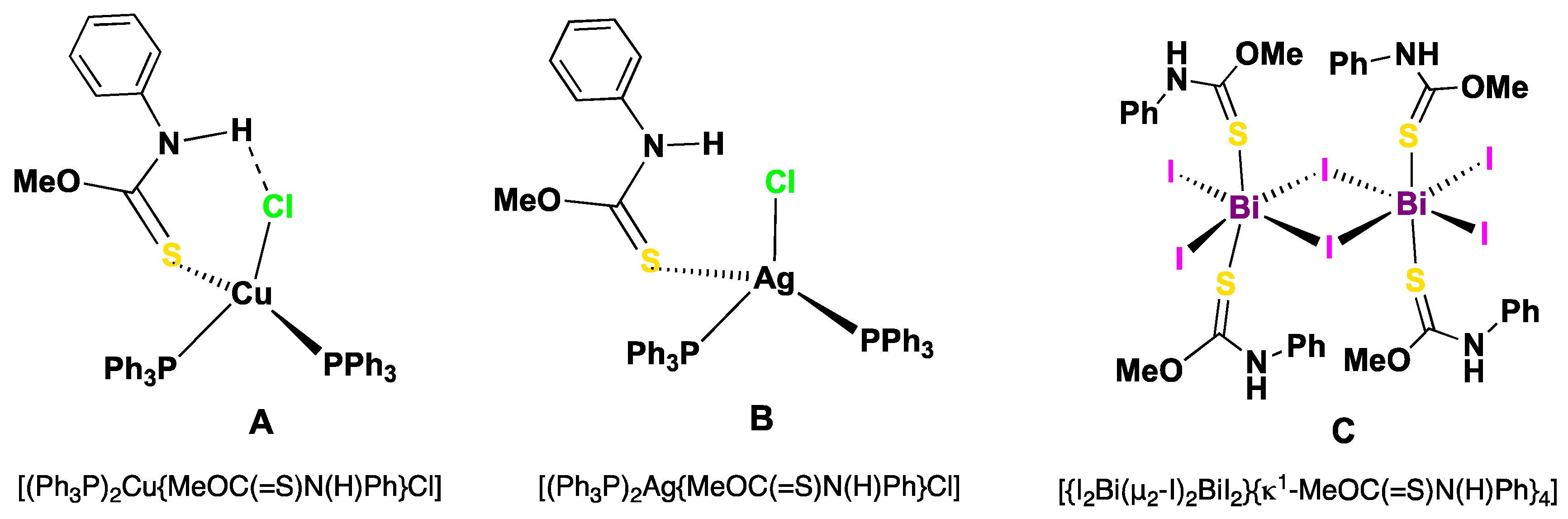
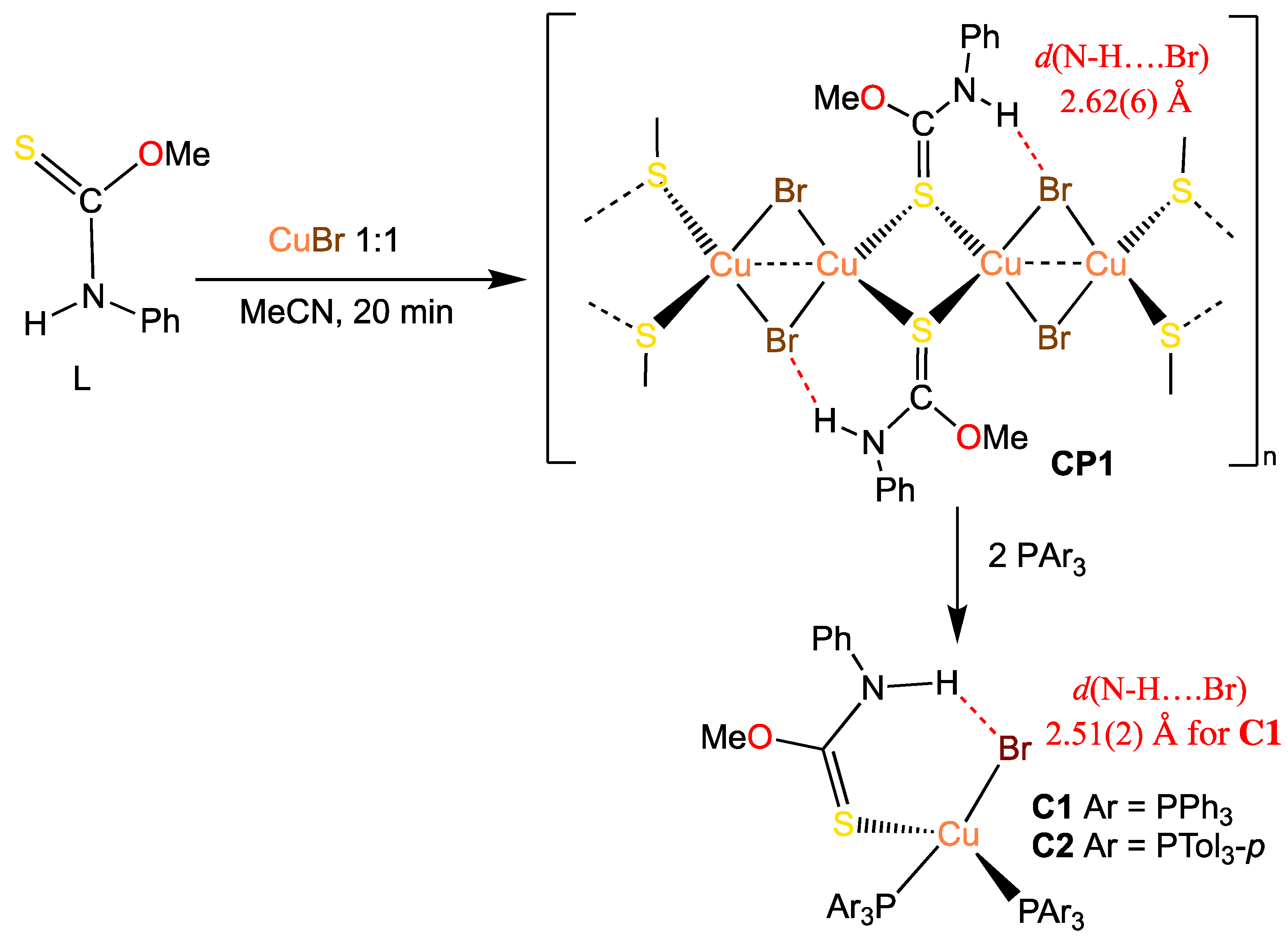
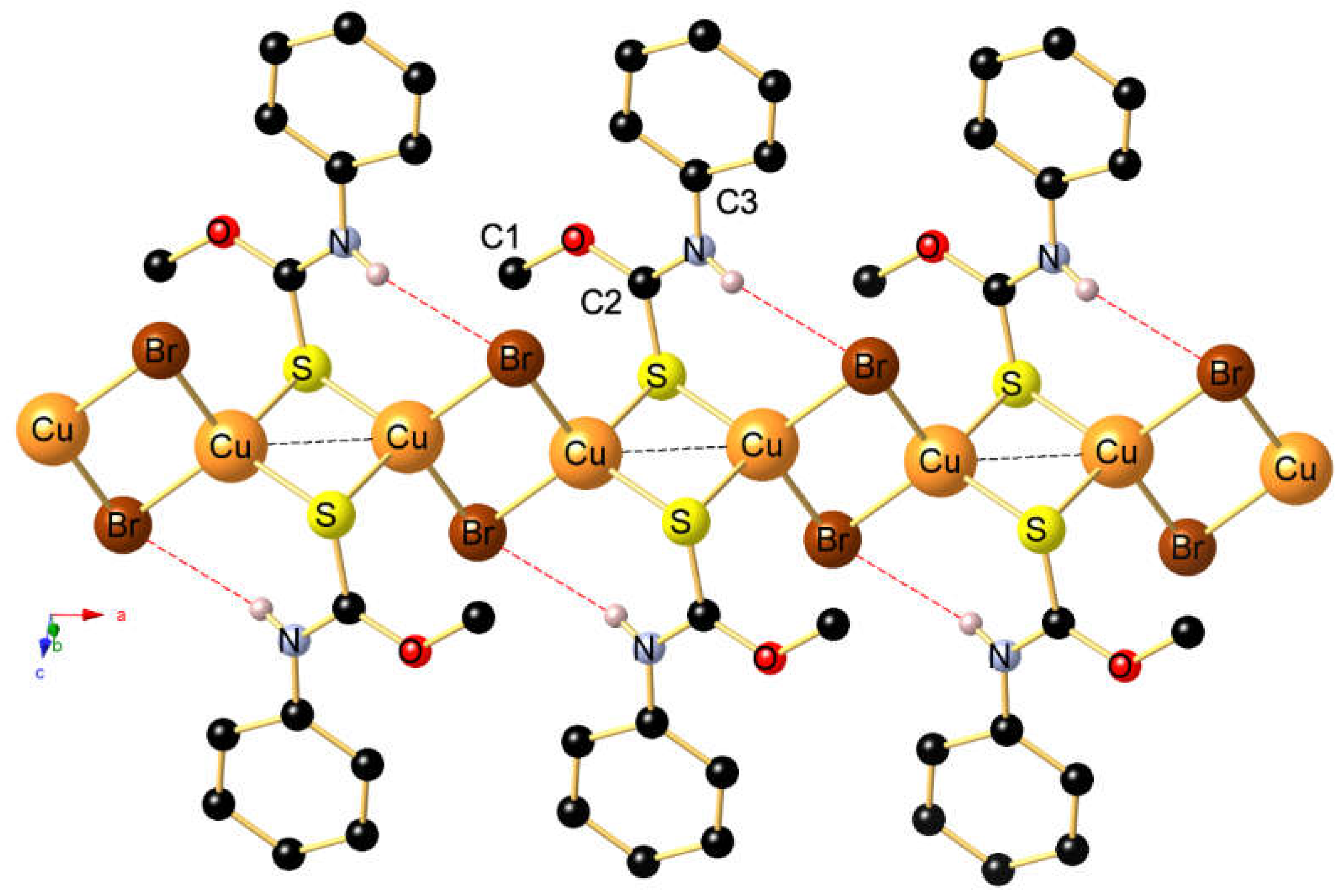
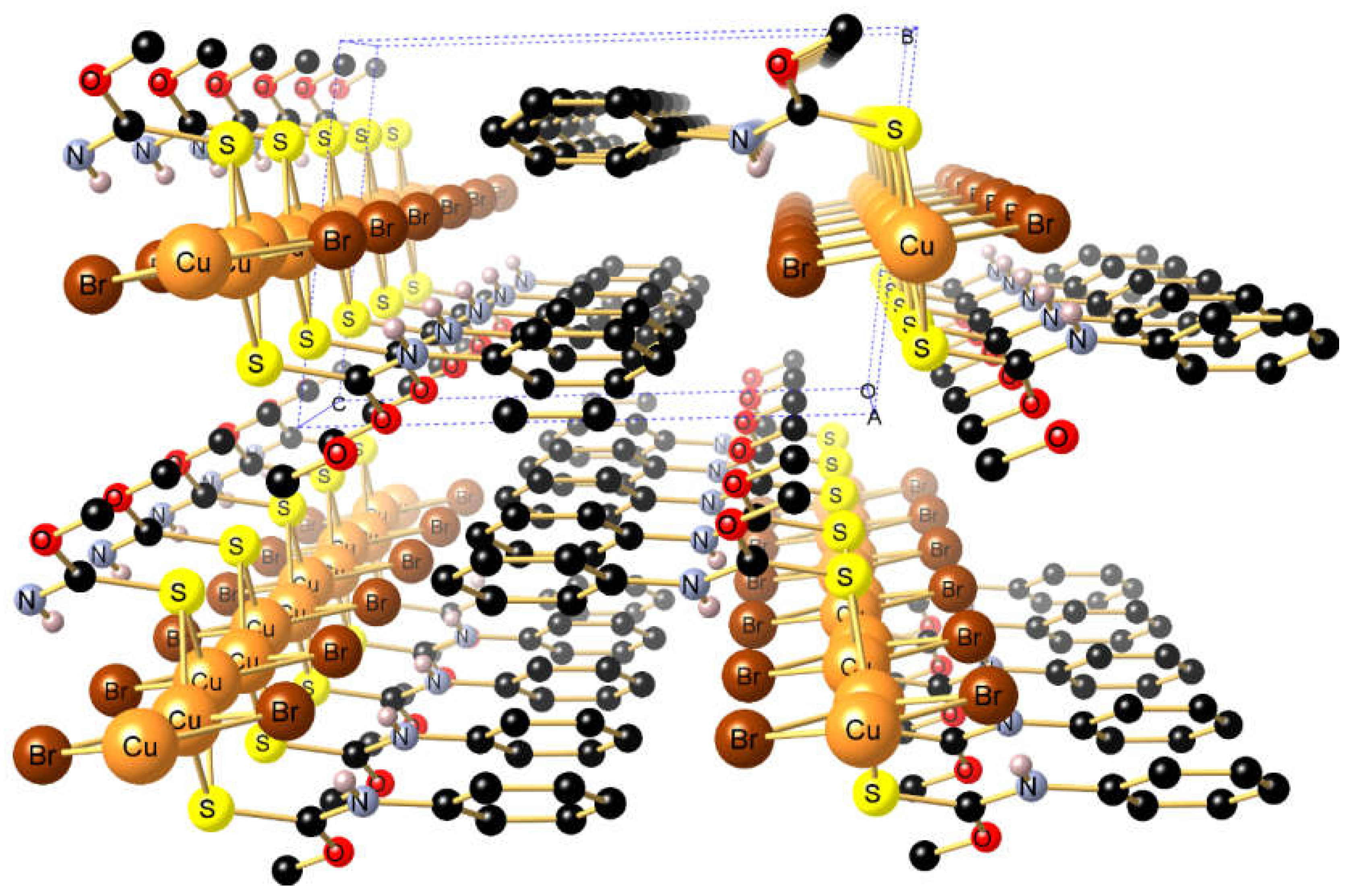
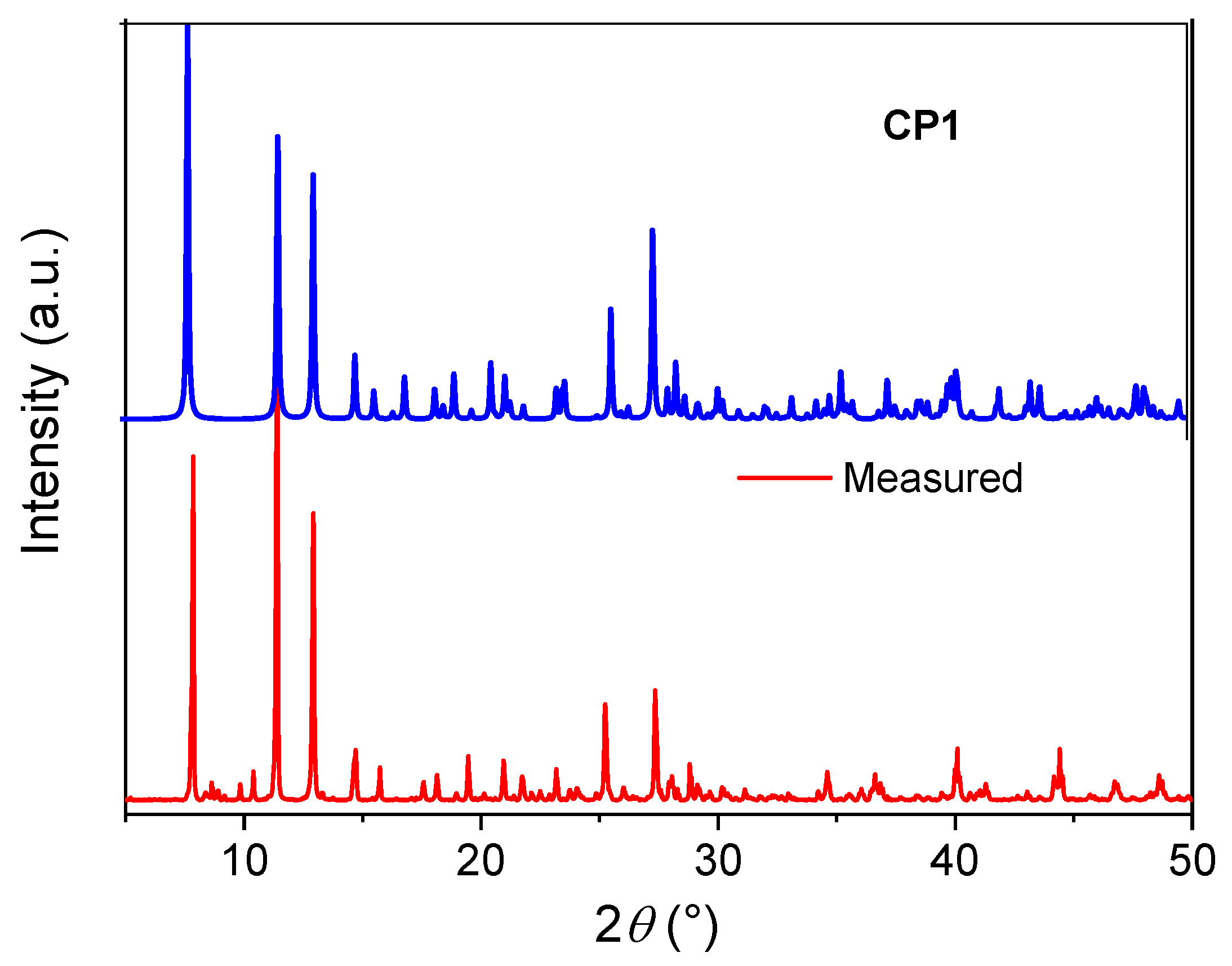
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Németh, A.G.; Keserű, G.M.; Ábrányi-Balogh, P. A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: A mild, catalyst-free approach towards O-thiocarbamates and dithiocarbamates. Beilstein J. Org. Chem. 2019, 15, 1523–1533. [Google Scholar] [PubMed]
- Zhang, J.; Zang, Q.; Yang, F.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Sulfur Conversion to Multifunctional Poly(O-thiocarbamate)s through Multicomponent Polymerizations of Sulfur, Diols, and Diisocyanides. J. Am. Chem. Soc. 2021, 143, 3944–3950. [Google Scholar] [CrossRef]
- Shadap, L.; Tyagi, J.L.; Poluri, K.M.; Novikov, S.; Timothy Lo, C.W.; Mozharivskyj, Y.; Kollipara, M.R. Insights to the strained thiocarbamate derivative complexes of platinum group metals induced by azide as a co-ligand:Characterization and biological studies. J. Organomet. Chem. 2020, 920, 121345. [Google Scholar] [CrossRef]
- Krátky, M.; Volková, M.; Novotná, E.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. Synthesis and biological activity of new salicylanilide N,N-disubstituted carbamates and thiocarbamates. Bioorg. Med. Chem. 2014, 22, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Le, C.; Yeung, Y.Y. Enantioselective bromolactonization of cis-1,2-disubstituted olefinic acids using an amino-thiocarbamate catalyst. Chem. Commun. 2012, 48, 5793–5795. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Edu. 1987, 64, 561–567. [Google Scholar] [CrossRef]
- Yeo, C.I.; Halim, S.N.A.; Weng Ng, S.; Tan, S.L.; Zukerman-Schpector, J.; Ferreira, M.A.B.; Tiekink, E.R.T. Investigation of putative arene-C–H···π(quasi-chelate ring) interactions in copper(I) crystal structures. Chem. Commun. 2014, 50, 5984–5986. [Google Scholar]
- Yeo, C.I.; Teow, S.Y.; Liew, L.Y.; Chew, J.; Tiekink, E.R.T. Crystal structure of chlorido-(O-methyl phenylcarbamothioamide-κS)-bis (triphenylphosphane-κP)silver(I), C44H39AgClNOP2S. Z. Kristallogr. NCS 2020, 235, 1473–1475. [Google Scholar] [CrossRef]
- Hall, V.J.; Siasios, G.; Tiekink, E.R.T. Trioganophosphinegold(I)carbonimidothioates. Aust. J. Chem. 1993, 46, 561–570. [Google Scholar] [CrossRef]
- Bandoli, G.; Clemente, D.A.; Sindellari, L.; Tondello, E. Preparation, properties, and crystal structure of dichlorobis-(O-ethyl thiocarbamate)mercury(II). J. Chem. Soc. Dalton Trans. 1975, 449–452. [Google Scholar] [CrossRef]
- Furlani, C.; Tarantelli, T.; Gastaldi, L.; Porta, P. Complexing behaviour of thiocarbamic esters: Crystal and molecular structure of bis-(O-methylphenylthiocarbamato) (triphenylphosphine)-palladium(II). J. Chem. Soc. A 1971, 3778–3783. [Google Scholar] [CrossRef]
- Arar, W.; Khatyr, A.; Knorr, M.; Strohmann, C.; Schmidt, A. Bis(µ-iodo)-tetrakis(O-methyl N-phenylthiocarbamate)-tetraiodo-dibismuth. Molbank 2022, 2022, M1381. [Google Scholar] [CrossRef]
- Arar, W.; Khatyr, A.; Knorr, M.; Brieger, L.; Krupp, A.; Strohmann, C.; Efrit, M.L.; Ben Akacha, A. Synthesis, crystal Structures and hirshfeld analyses of phosphonothioamidates (EtO)2P(=O)C(=S)N(H)R (R = Cy, Bz) and their coordination on CuI and HgX2 (X = Br, I). Phosphorus Sulfur. Silicon Relat. Elem. 2021, 196, 845–858. [Google Scholar] [CrossRef]
- Hameau, A.; Guyon, F.; Knorr, M.; Enescu, M.; Strohmann, C. Self-Assembly of Dithiolene-based Coordination Polymers of Mercury(II): Dithioether versus Thiocarbonyl Bonding. Monatsh. Chem. 2006, 137, 545–555. [Google Scholar] [CrossRef]
- Guyon, F.; Hameau, A.; Khatyr, A.; Knorr, M.; Amrouche, H.; Fortin, D.; Harvey, P.D.; Strohmann, C.; Ndiaye, A.L.; Huch, V.; et al. Syntheses, Structures, and Photophysical Properties of Mono- and Dinuclear Sulfur-Rich Gold(I) Complexes. Inorg. Chem. 2008, 47, 7483–7492. [Google Scholar] [CrossRef]
- Hameau, A.; Guyon, F.; Khatyr, A.; Knorr, M.; Strohmann, C. 4,5-Bis(methylthio)-1,3-dithiole-2-thione, a versatile sulphur-rich building block for the self-assembly of Cu(I) and Ag(I) coordination polymers: Dithioether versus thiocarbonyl bonding. Inorg. Chim. Acta 2012, 388, 60–70. [Google Scholar] [CrossRef]
- Arar, W.; Ben Ali, R.; El May, M.V.; Khatyr, A.; Jourdain, I.; Knorr, M.; Brieger, L.; Scheel, R.; Strohmann, C.; Chaker, A.; et al. Synthesis, crystal structures and biological activities of halogeno-(O-alkylphenylcarbamothioate)bis(triarylphosphine)copper (I) complexes. J. Mol. Struct. 2023, 1284, 135370. [Google Scholar] [CrossRef]
- Chou, W.N.; Pomerantz, M. N-phenyl-P,P,P-triarylphospha-λ5-azenes, triarylphosphines, and triarylphosphine oxides. Substituent effects on nitrogen-15, phosphorus-31, and carbon-13 NMR spectra. J. Org. Chem. 1991, 56, 2762–2769. [Google Scholar] [CrossRef]
- Hisao, N.; Morio, H.; Yoshihiko; Masahiroj, K.; Tsumoto, O. The Crystal Structure of Di-μ-Bromo-tris(triphenylphosphine)dicopper(II). Bull. Soc. Jpn. 1981, 54, 1247–1248. [Google Scholar] [CrossRef]
- Moussa, J.; Chamoreau, L.M.; Gullo, M.P.; Esposti, A.D.; Barbieri, A.; Amouri, H. Induced phosphorescence from Pt → Ag and Ag(I)⋯Ag(I) metallophilic interactions in benzenedithiolatodiimine-Pt2/Ag2 clusters: A combined experimental and theoretical investigation. Dalton Trans. 2016, 45, 2906–2913. [Google Scholar] [CrossRef]
- Benard, M.; Bodensieck, U.; Braunstein, P.; Knorr, M.; Strampfer, M.; Strohmann, C. Conformation ControI in Polymetaliic Mesocycles by Metal -Metal Bonding: The First Example of an Hg-Cu Interaction. Angew. Chem. Int. Ed. Engl. 1997, 36, 2758–2761. [Google Scholar]
- Harisomayajula, N.S.; Makovetskyi, S.; Tsai, Y.C. Cuprophilic Interactions in and between Molecular Entities. Chem. Eur. J. 2019, 25, 8936–8954. [Google Scholar] [CrossRef] [PubMed]
- Troyano, J.; Zapata, E.; Perles, J.; Amo-Ochoa, P.; Fernández-Moreira, V.; Martínez, J.I.; Zamora, F.; Delgado, S. Multifunctional Copper(I) Coordination Polymers with Aromatic Mono- and Ditopic Thioamides. Inorg. Chem. 2019, 58, 3290–3301. [Google Scholar] [CrossRef] [PubMed]
- Knorr, M.; Guyon, F.; Khatyr, A.; Strohmann, C.; Allain, M.; Aly, S.M.; Lapprand, A.; Fortin, D.; Harvey, P.D. Construction of(CuX)2n Cluster- Containing(X= Br, I; n = 1, 2) Coordination Polymers Assembled by Dithioethers ArS(CH2)mSAr (Ar = Ph, p-Tol; m = 3, 5): Effect of the Spacer Length, Aryl Group, and Metal-to-Ligand Ratio on the Dimensionality, Cluster Nuclearity, and the Luminescence Properties of the Metal– Organic Frameworks. Inorg. Chem. 2012, 18, 9917–9934. [Google Scholar]
- Lee, S.Y.; Park, S.; Lee, S.S. Copper(I) Silver(I), and Palladium(II) Complexes of a Thiaoxamacrocycle Displaying Unusual Topologies. Inorg. Chem. 2009, 48, 11335–11341. [Google Scholar] [CrossRef]
- Lucas, C.R.; Liang, W.; Miller, D.O.; Bridson, J.N. Metal Complexes of 1-Oxa-4,7-dithiacyclononane. Inorg. Chem. 1997, 36, 4508–4513. [Google Scholar] [CrossRef]
- Lobana, T.S.; Sharma, R.; Sharma, R.; Butcher, R.J. Metal Derivatives of Heterocyclic Thioamides: Synthesis and Crystal Structures of Copper Complexes with 1-Methyl-1,3-imidazoline-2-thione and 1,3-Imidazoline-2-thione. Z. Anorg. Allg. Chem. 2008, 634, 1785–1790. [Google Scholar] [CrossRef]
- Ho, S.Y.; Lai, C.S.; Tiekink, E.R.T. O-Methyl N-phenylthiocarbamate. Acta Cryst. 2003, E59, o1155–o1156. [Google Scholar]
- Sheldrick, G. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arar, W.; Viau, L.; Jourdain, I.; Knorr, M.; Strohmann, C.; Scheel, R.; Ben Akacha, A. Synthesis of Catena-bis(μ-bromo)-(O-methyl-N-phenylthiocarbamate)-dicopper(I) and Its Reactivity towards PAr3 (Ar = Ph, p-Tol). Molbank 2023, 2023, M1655. https://doi.org/10.3390/M1655
Arar W, Viau L, Jourdain I, Knorr M, Strohmann C, Scheel R, Ben Akacha A. Synthesis of Catena-bis(μ-bromo)-(O-methyl-N-phenylthiocarbamate)-dicopper(I) and Its Reactivity towards PAr3 (Ar = Ph, p-Tol). Molbank. 2023; 2023(2):M1655. https://doi.org/10.3390/M1655
Chicago/Turabian StyleArar, Wafa, Lydie Viau, Isabelle Jourdain, Michael Knorr, Carsten Strohmann, Rebecca Scheel, and Azaiez Ben Akacha. 2023. "Synthesis of Catena-bis(μ-bromo)-(O-methyl-N-phenylthiocarbamate)-dicopper(I) and Its Reactivity towards PAr3 (Ar = Ph, p-Tol)" Molbank 2023, no. 2: M1655. https://doi.org/10.3390/M1655
APA StyleArar, W., Viau, L., Jourdain, I., Knorr, M., Strohmann, C., Scheel, R., & Ben Akacha, A. (2023). Synthesis of Catena-bis(μ-bromo)-(O-methyl-N-phenylthiocarbamate)-dicopper(I) and Its Reactivity towards PAr3 (Ar = Ph, p-Tol). Molbank, 2023(2), M1655. https://doi.org/10.3390/M1655






