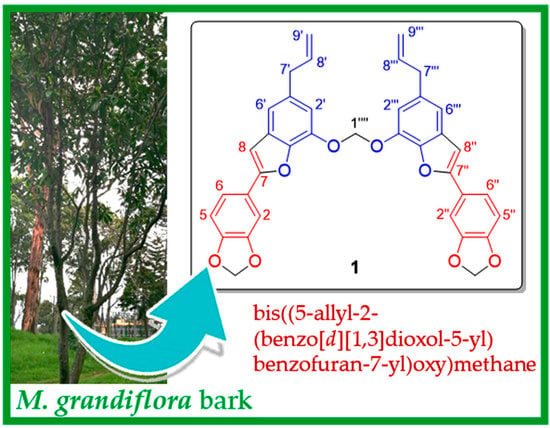
Bis((5-allyl-2-(benzo[d][1,3]dioxol-5-yl)benzofuran-7-yl)oxy)methane: An Unusual Nor-Neolignan Dimer from Magnolia grandiflora L.
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- ElGwedy, H.I.M.; Abido, A.I.A.; ElTorky, M.G.; Weheda, B.M.; Gaber, M.K.A. In Vitro Propagation and Ex Vitro Acclimatization of Magnolia (Magnolia grandiflora, Linn) Trees. J. Adv. Agric. Res. 2015, 20, 498–517. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Chemical Composition, Antioxidant Activity and Cytotoxicity on Tumour Cells of the Essential Oil from Flowers of Magnolia grandiflora Cultivated in Iran. Nat. Prod. Res. 2017, 31, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Sjöman, H.; Hirons, A.D.; Bassuk, N.L. Magnolias as Urban Trees–A Preliminary Evaluation of Drought Tolerance in Seven Magnolia Species. Arboric. J. 2018, 40, 47–56. [Google Scholar] [CrossRef]
- Bernal, F.A.; Matulevich, J.A.; Corredor, J.A.; Coy-Barrera, E. GC/MS-Based Fingerprinting Reveals Two Chemotypes in the Leaf Essential Oils from Magnolia grandiflora Trees within the Urban Forestry of a Colombian Andean Plateau. Chem. Biodivers. 2022, 19, e202200448. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.D. Use of Magnolia (Magnolia grandiflora) Seeds in Medicine, and Possible Mechanisms of Action. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 727–732. ISBN 978-0-12-375688-6. [Google Scholar]
- Lim, T.K. Magnolia Grandiflora. In Edible Medicinal and Non Medicinal Plants: Volume 8, Flowers; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 243–275. ISBN 978-94-017-8748-2. [Google Scholar]
- Latif, A.; Du, Y.; Dalal, S.R.; Fernández-Murga, M.L.; Merino, E.F.; Cassera, M.B.; Goetz, M.; Kingston, D.G.I. Bioactive Neolignans and Other Compounds from Magnolia grandiflora L.: Isolation and Antiplasmodial Activity. Chem. Biodivers. 2017, 14, e1700209. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Kumarihamy, M.; Chaturvedi, K.; Ibrahim, M.A.M.; Lambert, J.A.; Godfrey, M.; Doerksen, R.J.; Muhammad, I. In Vitro and In Silico Studies of Neolignans from Magnolia grandiflora L. Seeds against Human Cannabinoids and Opioid Receptors. Molecules 2023, 28, 1253. [Google Scholar] [CrossRef] [PubMed]
- Schühly, W.; Khan, S.I.; Fischer, N.H. Neolignans from North American Magnolia Species with Cyclooxygenase 2 Inhibitory Activity. Inflammopharmacology 2009, 17, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Yamamura, M.; Nakatsubo, T.; Suzuki, S.; Hattori, T. Stereoselectivity of the Biosynthesis of Norlignans and Related Compounds. In The Biological Activity of Phytochemicals; Gang, D.R., Ed.; Springer: New York, NY, USA, 2011; pp. 179–197. ISBN 978-1-4419-7299-6. [Google Scholar]
- Moss, G.P. Nomenclature of Lignans and Neolignans (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Veitch, N.C. A 2-Arylbenzofuran from Roots of Cicer bijugum Associated with Fusarium Wilt Resistance. Phytochemistry 1998, 48, 947–951. [Google Scholar] [CrossRef]
- Chérigo, L.; Polanco, V.; Ortega-Barria, E.; Heller, M.V.; Capson, T.L.; Rios, L.C. Antitrypanosomal Activity of a Novel Norlignan Purified from Nectandra lineata. Nat. Prod. Res. 2005, 19, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Puentes De Díaz, A.M. Neolignans from Anaxagorea clavata. Phytochemistry 1997, 44, 345–346. [Google Scholar] [CrossRef]
- Rios-Motta, J.; Avella, E. 2-Arylbenzofuran Neolignans from the Bark of Nectandra purpurascens (Lauraceae). Nat. Prod. Commun. 2010, 5, 1934578X1000500716. [Google Scholar] [CrossRef]
- Coy, E.; Cuca, L.E. Novel Oxoaporphine Alkaloid and Other Chemical Constituents Isolated from Pleurothyrium cinereum (Lauraceae). Rev. Colomb. Química 2008, 37, 127–134. [Google Scholar]
- Pal, G.; Venkateswaran, R. V Synthesis of Neolignans from Anaxagorea clavata. J. Chem. Res. 2003, 2003, 142–143. [Google Scholar] [CrossRef]
- Li, H.-M.; Zhao, S.-R.; Huo, Q.; Ma, T.; Liu, H.; Lee, J.k.; Hong, Y.-S.; Wu, C.-Z. A New Dimeric Neolignan from Magnolia grandiflora L. Seeds. Arch. Pharm. Res. 2015, 38, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Marentes-Culma, R.; Orduz-Díaz, L.L.; Coy-Barrera, E. Targeted Metabolite Profiling-Based Identification of Antifungal 5-n-Alkylresorcinols Occurring in Different Cereals against Fusarium oxysporum. Molecules 2019, 24, 770. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.N.; Stevenson, P.C.; Kokubun, T.; Hall, D.R. Antibacterial and Antifungal Activity of Cicerfuran and Related 2-Arylbenzofurans and Stilbenes. Microbiol. Res. 2009, 164, 191–195. [Google Scholar] [CrossRef] [PubMed]
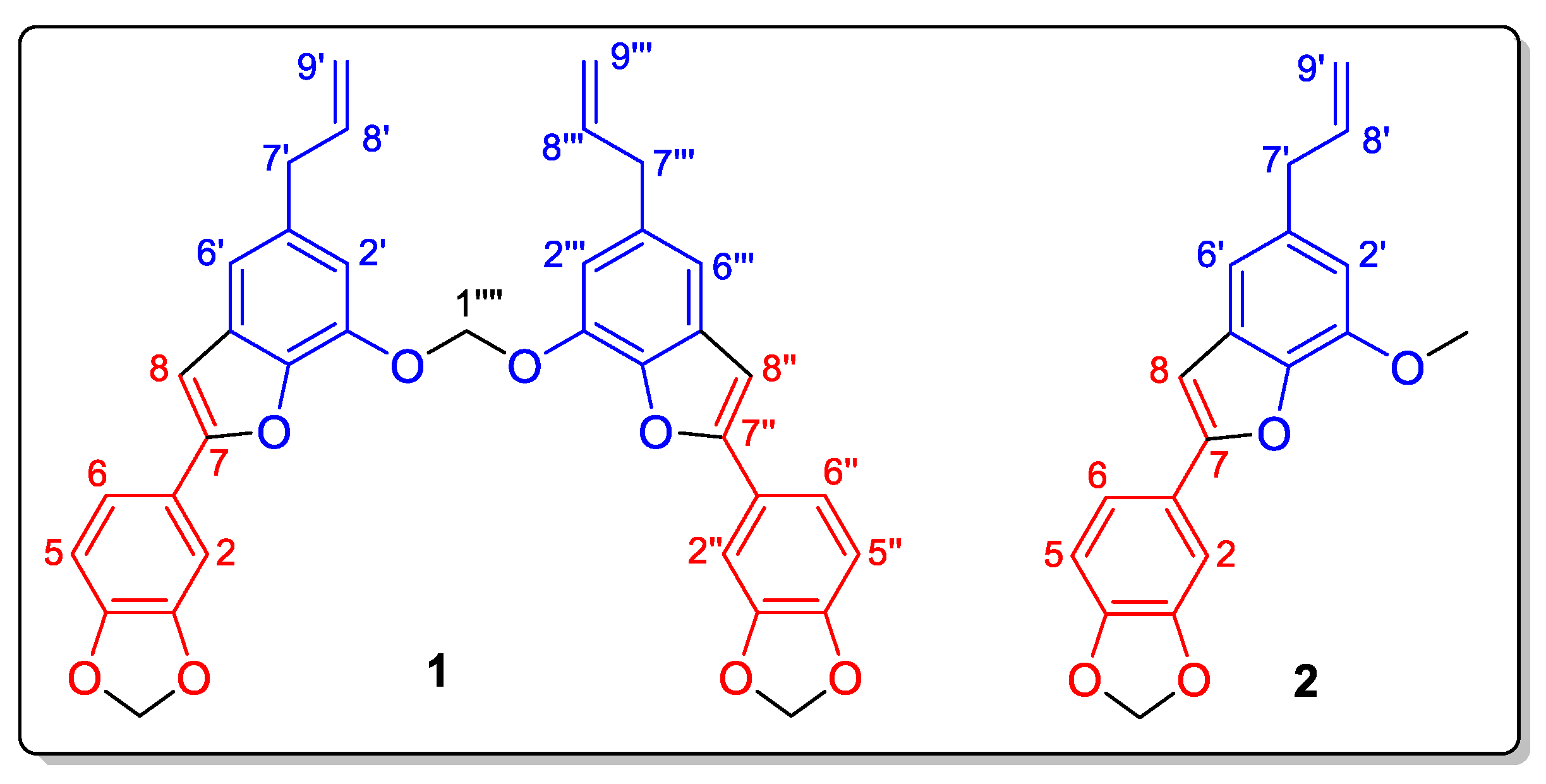
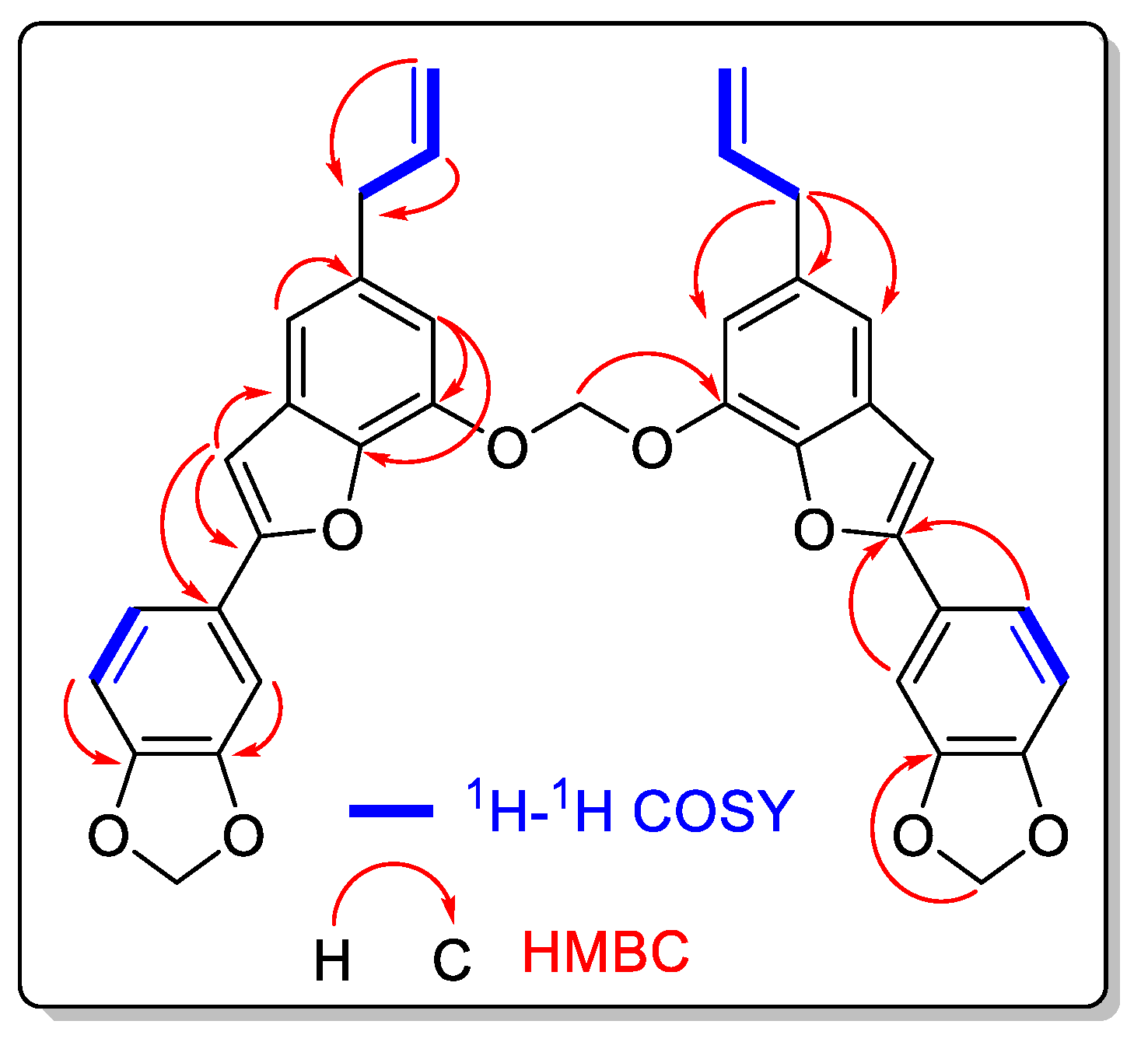
| Position | Type | δC | δH (multpl., J (Hz), Integral) |
|---|---|---|---|
| 1, 1″ | C × 2 | 124.8 | |
| 2, 2″ | CH × 2 | 105.5 | 7.36, (d, J = 1.8, 1H) |
| 3, 3″ | C × 2 | 148.1 | |
| 4, 4″ | C × 2 | 148.2 | |
| 5, 5″ | CH × 2 | 108.6 | 6.89, (d, J = 8.1, 1H) |
| 6, 6″ | CH × 2 | 119.2 | 7.43, (dd, J = 8.1, 1.8, 1H) |
| 7, 7″ | C × 2 | 156.1 | |
| 8, 8″ | CH × 2 | 100.5 | 6.78, (s, 1H) |
| 1′, 1′′′ | C × 2 | 135.8 | |
| 2′, 2′′′ | CH × 2 | 107.6 | 6.98, (d, J = 1.5, 1H) |
| 3′, 3′′′ | C × 2 | 144.9 | |
| 4′, 4′′′ | C × 2 | 142.7 | |
| 5′, 5′′′ | C × 2 | 131.2 | |
| 6′, 6′′′ | CH × 2 | 112.7 | 6.68, (d, J = 1.5, 1H) |
| 7′, 7′′′ | CH2 × 1 | 40.6 | 3.50, (d, J = 6.7, 2H) |
| 8′, 8′′′ | CH × 1 | 138.1 | 6.11, (ddt, J = 16.8, 10.1, 6.7, 1H) |
| 9′, 9′′′ | CH2 × 2 | 115.7 | 5.26–5.14, (ddt, J = 10.3, 1.9, 1.2, 2H) |
| 1′′′′ | CH2 | 89.7 | 5.59, (s, 2H) |
| OCH2O | CH2 × 2 | 101.4 | 5.99, (s, 2H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas-Laverde, D.; Quiroga, D.; Coy-Barrera, E. Bis((5-allyl-2-(benzo[d][1,3]dioxol-5-yl)benzofuran-7-yl)oxy)methane: An Unusual Nor-Neolignan Dimer from Magnolia grandiflora L. Molbank 2023, 2023, M1612. https://doi.org/10.3390/M1612
Cárdenas-Laverde D, Quiroga D, Coy-Barrera E. Bis((5-allyl-2-(benzo[d][1,3]dioxol-5-yl)benzofuran-7-yl)oxy)methane: An Unusual Nor-Neolignan Dimer from Magnolia grandiflora L. Molbank. 2023; 2023(2):M1612. https://doi.org/10.3390/M1612
Chicago/Turabian StyleCárdenas-Laverde, Diego, Diego Quiroga, and Ericsson Coy-Barrera. 2023. "Bis((5-allyl-2-(benzo[d][1,3]dioxol-5-yl)benzofuran-7-yl)oxy)methane: An Unusual Nor-Neolignan Dimer from Magnolia grandiflora L." Molbank 2023, no. 2: M1612. https://doi.org/10.3390/M1612
APA StyleCárdenas-Laverde, D., Quiroga, D., & Coy-Barrera, E. (2023). Bis((5-allyl-2-(benzo[d][1,3]dioxol-5-yl)benzofuran-7-yl)oxy)methane: An Unusual Nor-Neolignan Dimer from Magnolia grandiflora L. Molbank, 2023(2), M1612. https://doi.org/10.3390/M1612