(Z)-5-Benzylidene-4-phenyl-2-(p-tolyl)-4,5-dihydrooxazole
Abstract
:1. Introduction
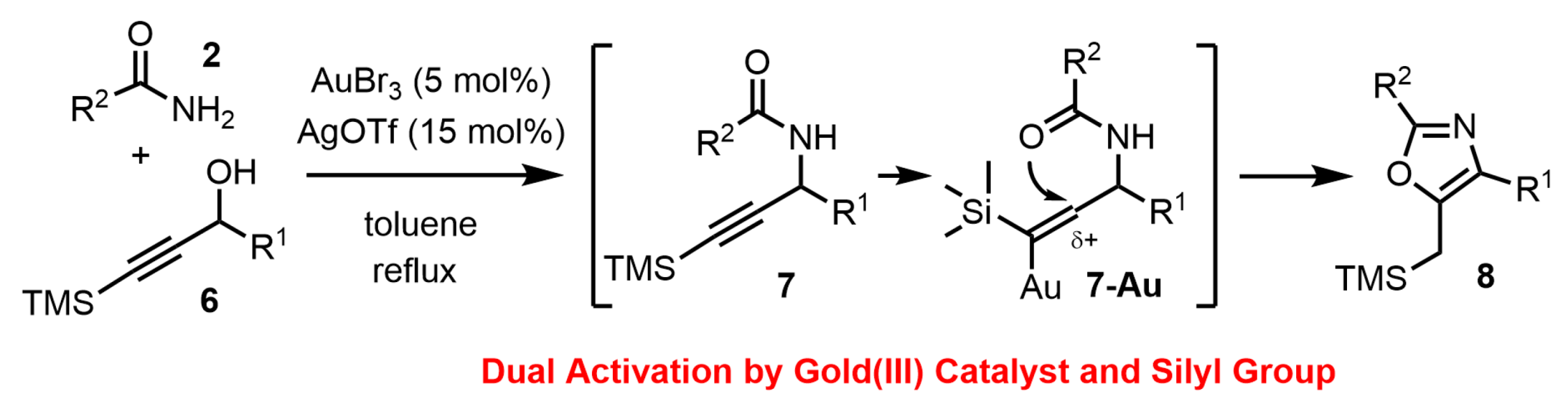
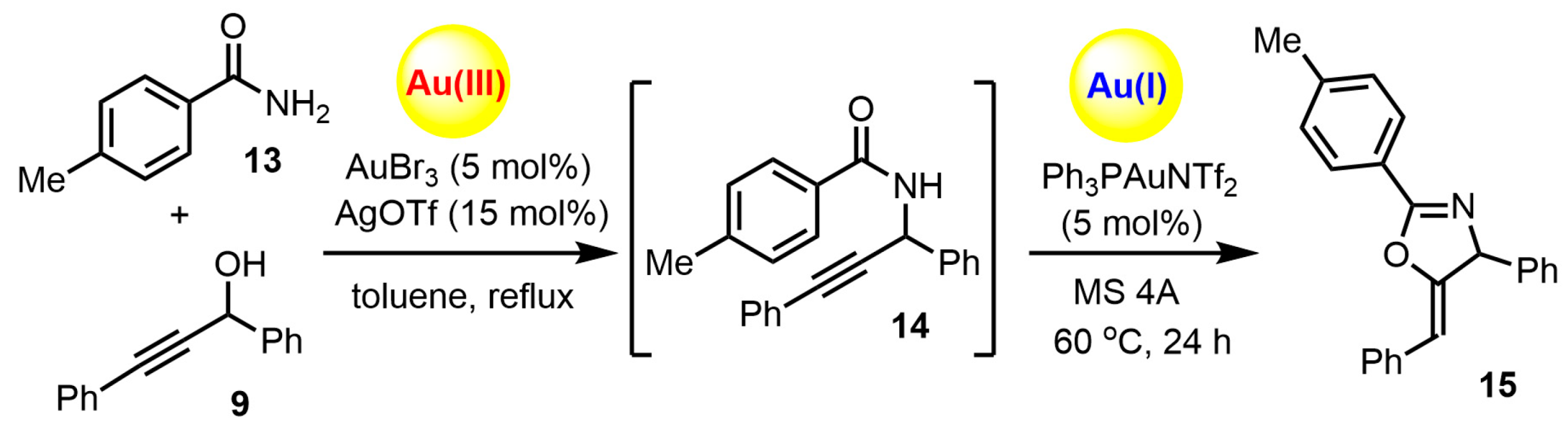
2. Results and Discussion
2.1. Chemistry
2.2. X-ray Structure Analysis
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (Z)-5-benzylidene-4-phenyl-2-(p-tolyl)-4,5-dihydrooxazole (15)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yeh, V.S.C. Recent advances in the total synthesis of oxazole-containing natural products. Tetrahedron 2004, 60, 11995–12042. [Google Scholar]
- Wipf, P. Synthetic studies of biologically active marine cyclopeptides. Chem. Rev. 1995, 95, 2115–2134. [Google Scholar] [CrossRef]
- Lipshulz, B.H. Five-membered heteroatomic rings as intermediates in organic synthesis. Chem. Rev. 1986, 86, 795–819. [Google Scholar] [CrossRef]
- Wipf, P.; Venkatraman, S. From aziridines to oxazolines and thiazolines: The heterocyclic route to thiangazole. Synlett 1997, 1997, 1–10. [Google Scholar] [CrossRef]
- Dong, K.; Gurung, R.; Xu, X.; Doyle, M.P. Enantioselective catalytic cyclopropanation-rearrangement approach to chiral spiroketals. Org. Lett. 2021, 23, 3955–3959. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, S.; Tomkinson, N.C.O. Transition metal-mediated synthesis of oxazoles. Heterocycles 2014, 89, 2479–2542. [Google Scholar]
- Senadi, G.C.; Hu, W.-P.; Hsiao, J.-S.; Vandavasi, J.K.; Chen, C.-Y.; Wang, J.-J. Facile, selective, and regiocontrolled synthesis of oxazolines and oxazoles mediated by ZnI2 and FeCl3. Org. Lett. 2012, 14, 4478–4481. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Sato, D.; Yokomura, A.; Zhdankin, V.V.; Saito, A. Iodine(III)-mediated/catalyzed cycloisomerization-amination sequence of N-propargyl carboxamides. Adv. Synth. Catal. 2017, 359, 3243–3247. [Google Scholar] [CrossRef]
- Yu, X.; Xin, X.; Wan, B.; Li, X. Base-catalyzed cyclization of N-sulfonyl propargylamines to sulfonylmethyl-substituted oxazoles via sulfonyl migration. J. Org. Chem. 2013, 78, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Milton, M.D.; Inada, Y.; Nishibayashi, Y.; Uemura, S. Ruthenium- and gold-catalyzed sequential reactions: A straightforward synthesis of substituted oxazoles from propargylic alcohols and amides. Chem. Commun. 2004, 23, 2712–2713. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.P.; Liu, R.-S. Zn(OTf)2-catalyzed cyclization of proparyl alcohols with anilines, phenols, and amides for synthesis of indoles, benzofurans, and oxazoles through different annulation mechanisms. J. Org. Chem. 2006, 71, 4951–4955. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-M.; Zheng, F.-J.; Lin, H.-X.; Zhan, Z.-P. Bronsted acid-catalyzed propargylation/cycloisomerization tandem reaction: One-pot synthesis of substituted oxazoles from propargylic alcohols and amides. J. Org. Chem. 2009, 74, 3148–3151. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Yasuda, A.; Shibata, M.; Ban, S.; Hashimoto, Y.; Okamoto, I.; Tamura, O. Gold(I)/(III)-catalyzed synthesis of cyclic ethers; valency-controlled cyclization modes. Org. Lett. 2015, 17, 2668–2671. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Tsunokake, T.; Narikiyo, Y.; Harada, M.; Tachibana, T.; Saito, Y.; Ban, S.; Hashimoto, Y.; Okamoto, I.; Tamura, O. Gold(I)/(III)-catalyzed synthesis of 2-substituted piperidines; valency-controlled cyclization modes. Tetrahedron Lett. 2015, 56, 6269–6272. [Google Scholar] [CrossRef]
- Morita, N.; Saito, Y.; Muraji, A.; Ban, S.; Hashimoto, Y.; Okamoto, I.; Tamura, O. Gold-catalyzed synthesis of 2-substituted azepanes; strategic use of soft gold(I) and hard gold(III) catalysts. Synlett 2016, 27, 1936–1940. [Google Scholar] [CrossRef]
- Morita, N.; Miyamoto, M.; Yoda, A.; Yamamoto, M.; Ban, S.; Hashimoto, Y.; Tamura, O. Gold-catalyzed dehydrative Friedel-Crafts reaction and Nazarov cyclization sequence: An efficient synthesis of 1,3-diarylindenes from propargylic alcohols. Tetrahedron Lett. 2016, 57, 4460–4463. [Google Scholar] [CrossRef]
- Morita, N.; Oguro, K.; Takahashi, S.; Kawahara, M.; Ban, S.; Hashimoto, Y.; Tamura, O. Gold(III)-catalyzed synthesis of 2,3,4-trisubstituted dihydropyrans from propargylic alcohols with 1,3-dicarbonyl compounds. Heterocycles 2017, 95, 172–180. [Google Scholar]
- Morita, N.; Sano, A.; Sone, A.; Aonuma, S.; Matsunaga, A.; Hashimoto, Y.; Tamura, O. Efficient one-pot synthesis of substituted oxazoles from 3-trimethylsilylpropargylic alcohols and amides by gold-catalyzed substitution followed by cycloisomerization. Heterocycles 2018, 97, 719–728. [Google Scholar]
- Weyrauch, J.P.; Hashmi, A.S.K.; Schuster, A.; Hengst, T.; Schetter, S.; Littmann, A.; Rudolph, M.; Hamzic, M.; Visus, J.; Rominger, F.; et al. Cyclization of propargylic amides: Mild access to oxazole derivatives. Chem. Eur. J. 2010, 16, 956–963. [Google Scholar] [CrossRef] [PubMed]
- CCDC 2239857. Contains the Supplementary Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 4 February 2023).



| Parameter | Data |
|---|---|
| Identification code | C23H19NO |
| Formula weight | 325.29 |
| Temperature/K | 293(2) |
| Crystal system | triclinic |
| Space group | P-1 |
| Unit cell dimensions | a/Å 8.0541(4) α/o 81.010(4) |
| b/Å 9.3301 (5) β/o 89.182(4) | |
| c/Å 11.8454(6) γ/o 72.271(5) | |
| Volume/Å3 | 836.91(8) |
| Z | 2 |
| ρcalc g/cm3 | 1.291 |
| μ/mm−1 | 0.611 |
| F(000) | 344.0 |
| Crystal size/mm-1 | 0.25 × 0.15 × 0.20 |
| Radiation | Cu Kα (λ = 1.54184) |
| 2Θ range for data collection/° | 16.83 to 102.658 |
| Index ranges | −8 ≤ h ≤ 8, −9 ≤ k ≤ 9, −5 ≤ l ≤ 11 |
| Reflections collected | 1752 |
| Independent reflections | 1462 [Rint = 0.0045, Rsigma = 0.0121] |
| Data/restraints/parameters | 1462/0/227 |
| Goodness-of-fit on F2 | 1.056 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0301, wR2 = 0.0781 |
| Final R indexes [all data] | R1 = 0.0316, wR2 = 0.0793 |
| Largest diff. peak/hole/e Å−3 | 0.17/−0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, N.; Chiaki, H.; Aonuma, S.; Tanaka, K., III; Hashimoto, Y.; Tamura, O. (Z)-5-Benzylidene-4-phenyl-2-(p-tolyl)-4,5-dihydrooxazole. Molbank 2023, 2023, M1600. https://doi.org/10.3390/M1600
Morita N, Chiaki H, Aonuma S, Tanaka K III, Hashimoto Y, Tamura O. (Z)-5-Benzylidene-4-phenyl-2-(p-tolyl)-4,5-dihydrooxazole. Molbank. 2023; 2023(1):M1600. https://doi.org/10.3390/M1600
Chicago/Turabian StyleMorita, Nobuyoshi, Hitomi Chiaki, Shino Aonuma, Kosaku Tanaka, III, Yoshimitsu Hashimoto, and Osamu Tamura. 2023. "(Z)-5-Benzylidene-4-phenyl-2-(p-tolyl)-4,5-dihydrooxazole" Molbank 2023, no. 1: M1600. https://doi.org/10.3390/M1600
APA StyleMorita, N., Chiaki, H., Aonuma, S., Tanaka, K., III, Hashimoto, Y., & Tamura, O. (2023). (Z)-5-Benzylidene-4-phenyl-2-(p-tolyl)-4,5-dihydrooxazole. Molbank, 2023(1), M1600. https://doi.org/10.3390/M1600








