Abstract
The novel compound 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one is obtained in excellent yield via a thionation of the corresponding oxygen analogue. The product is isolated in pure form using column chromatography and is characterised using 1D and 2D NMR experiments, ATR IR and HRMS spectra, and single-crystal XRD.
1. Introduction
The isolation and separation of metal ions, especially critical ones, both from natural raw materials and from industrial waste, are among the main priorities of the world economy [1,2,3]. One of the most powerful tools in this direction is extraction processes [4,5,6,7,8,9,10,11,12]. The correct selection of ligands for each specific object is a major factor in achieving high efficiency and, therefore, the efforts of a huge number of scientific groups are directed towards the development of new effective organic molecules [13,14,15,16,17,18]. β-Dicarbonyl compounds are amongst the most widely used chelating extractants due to their excellent coordination properties [19,20,21,22,23,24]. A leading role inside the group belongs to acylpyrazolones because of the remarkable effectiveness displayed [25,26,27,28,29,30]. From the other side, sulphur-containing ligands possess spectacular coordination abilities [31,32,33,34] with extraordinarily broad applications [35,36,37,38,39,40,41,42,43].
As a part of our study on the development of effective ligands for metal isolation and separation [44,45,46,47,48,49,50], we focused our efforts towards the preparation of sulphur analogues of acylpyrazolones. The direct thioacylation is restricted by the very limited variety of reagents available on the market. However, numerous protocols for the direct construction of thio-ligands and for oxygen replacement in already-built molecules are developed [51,52,53,54], with the use of Lawesson’s reagent being amongst the most exploited in the latter [55,56,57,58,59,60,61]. Thiopyrazolones, in particular, are very poorly studied. To the best of our knowledge, there are only a few records in the literature including the C-thionation of pyrazolones [62], oxygen replacement leading to thiopyrazolones [63,64,65,66], and the preparation of thioacetyl pyrazolones through the direct acylation of a pyrazolone [67] or the thionation of 4-benzoyl-5-chloro-pyrazole [63].
Herein, we report on the synthesis of a novel ligand, 3-methyl-1-phenyl-4-thioacetylpyrazol-2-one, and its characterization with NMR and HRMS spectra in solution and by an ATR IR spectrum and single-crystal XRD in the solid state.
2. Results and Discussion
2.1. Synthesis
The novel compound 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one was obtained via the thionation of the corresponding acylpyrazolone, as shown in Scheme 1. The replacement of the acyl group oxygen by sulphur was carried out by using Lawesson’s reagent. The solvent, temperature, reaction prolongation, and reagent proportions were varied and the best conversion was obtained in toluene at 150 °C in a closed vessel. The pure product was isolated using column chromatography in 94% yield. It has to be noted that the monothionated compound was the only reaction product even when the transformation was performed by using an equimolar reagens ratio. The latter indicates that the tautomeric hydroxyl oxygen in 4-acetyl-3-methyl-1-phenyl-pyrazol-5-one cannot be replaced by sulphur in these particular conditions.
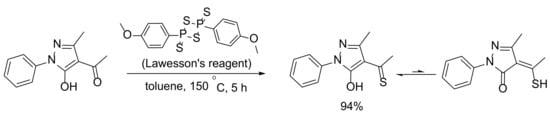
Scheme 1.
Synthesis of 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one.
The structure of the product was assigned using 1D and 2D NMR spectra (see Supporting Information). The 1H spectrum in CDCl3 showed characteristic signals for a phenyl group, two singlets for methyl groups, and a singlet for tautomeric OH in the low field. The 13C spectrum displayed signals for a phenyl group CH, two methyl groups, and five quaternary carbons. The latter were assigned by analysing the specific correlations in the HMBC experiment, namely, Cq-4, i-Ph, Cq-3, Cq-5, and C=S at 114.75, 137.04, 147.06, 160.59, and 216.85 ppm, respectively. The assignment of the signals for both methyl groups, CH3-3 at 2.563 and 17.48 and CH3-CS at 2.946 and 36.34 ppm, was based on the observed CH3-3/Cq-3, CH3-3/Cq-4, CH3-CS/Cq-4, and CH3-CS/C=S interactions. The structure of the product was confirmed using a high-resolution electrospray mass spectrum.
2.2. Crystallography
The synthesized 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one crystallized as orange-coloured crystals in a block shape, obtained using slow evaporation from iso-propanol. The title compound crystallized in the monoclinic C2/c space group with cell parameters a = 17.9610 (6) Å, b = 4.9709 (2) Å, c = 26.2901 (8) Å and β = 102.357 (2)° (Table S1). The unit cell contained a total of eight molecules (Z = 8, Z′ = 1), occupying a volume of 2292.86 (14) Å3. A close inspection of the molecular features of the 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one molecule revealed that it was almost planar with an RMSD of 0.035 Å, i.e., the conjugation was extremely pronounced (Figure 1). The planarity and molecular geometry were further stabilized by two intramolecular interactions (Table S2) C10-H10…O1 and O1-H1…S1. The three-dimensional packing of the 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one molecules was stabilized by CH3…π and C-H…S interactions (Figure 2).
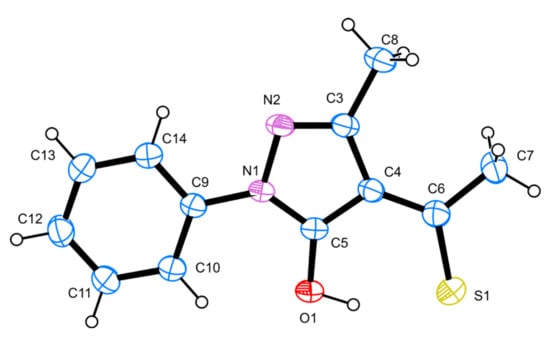
Figure 1.
A representation of the 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one molecule present in the asymmetric unit with employed numbering scheme; atomic displacement parameters (ADP) are at 50%, and hydrogen atoms are shown as small spheres with arbitrary radii.
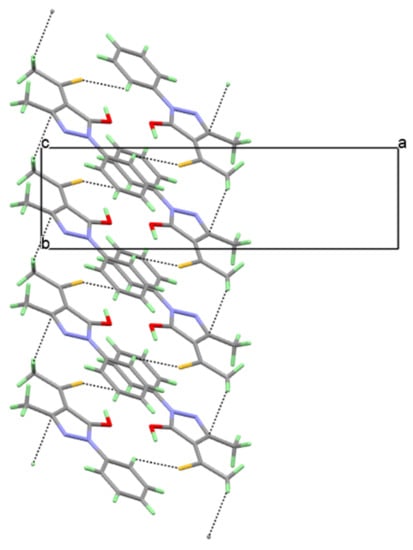
Figure 2.
Observed weak C-H3…π, C-H2 and C-H…S interactions which stabilize the crystal packing of 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one.
A search in the CSD (2022.3) for the thioacetylpyrazol-5-one moiety disclosed one similar structure (OCEGEJ, private communication), namely 3-methyl-1-phenyl-4-thiobenzoylpyrazol-5-one. The bond length values S1–C6, C5–O1, C4–C5, C4–C6, and C3–C4, of OCEGEJ and the title compound were comparable: 1.680 vs. 1.662 (2); 1.312 vs. 1.315 (2); 1.409 vs. 1.407 (3); 1.412 vs. 1.412 (3); and 1.443 vs. 1.437 (3) Å, respectively. The observed CS bond length in similar compounds is usually longer, e.g., 1.729 and 1.724 Å for UZIYIN and EZIXOC, respectively.
3. Materials and Methods
3.1. General
All reagents were purchased from Aldrich, Merck, and Fluka and were used without any further purification. The deuterated chloroform was purchased from Deutero GmbH (Kastellaun, Germany). Fluka silica gel (TLC-cards 60778 with fluorescent indicator 254 nm) plates were used for TLC chromatography and Rf-value determination. Merck Silica gel 60 (0.040–0.063 mm) was used for the flash chromatography purification of the product. The melting point was determined in a capillary tube on an SRS MPA100 OptiMelt (Sunnyvale, CA, USA) automated melting point system with a heating rate of 1 °C per min. The NMR spectra were recorded on a Bruker Avance NEO 400 spectrometer (Rheinstetten, Germany) in CDCl3; the chemical shifts were quoted in ppm in δ-values against tetramethylsilane (TMS) as an internal standard and the coupling constants were calculated in Hz. The assignment of the signals was confirmed by applying two-dimensional HSQC and HMBC techniques. The spectra were processed with the Topspin 3.6.3 program. The IR spectrum was measured on a Shimadzu IR Spirit FT-IR spectrometer (Shimadzu Corporation, Columbia, MD, USA) using QATR-S as a single-reflection ATR measurement attachment. The mass spectrum was recorded in positive mode on Q Exactive Plus Hybrid Quadrupole-Orbitrap Mass Spectrometer Thermo Scientific (ESI HR-MS) The spectrum was processed with the Xcalibur Free Style program version 4.5 (Thermo Fisher Scientific Inc., Waltham, MA, USA).
3.2. Synthesis of 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one
A solution of 3-methyl-1-phenyl-4-acetylpyrazol-5-one (2 mmol) and Lawesson’s reagent (1 mmol) in toluene (15 mL) was stirred at 150 °C in a closed vessel for 5 h. The solvent was evaporated in vacuo and the crude product was purified using column chromatography on silica gel by using DCM as a mobile phase to give the pure product: 94% yield; Rf 0.67 (1% acetone in DCM); orange solid; m. p. 61.8–62.1 °C; 1H NMR 2.563 (s, 3H, CH3-3), 2.946 (s, 3H, CH3-CS), 7.326 (t, 1H, J 7.3, p-Ph), 7.471 (dd, 2H, J 7.7, 7.4, m-Ph), 7.848 (d, 2H, J 7.7, o-Ph), 14.440 (s, 1H, OH); 13C NMR 17.48 (CH3-3), 36.34 (CH3-CS), 114.75 (Cq-4), 121.41 (o-Ph), 127.11 (p-Ph), 129.14 (m-Ph), 137.04 (i-Ph), 147.06 (Cq-3), 160.59 (Cq-5), 216.85 (C=S); IR (ATR) 1592, 1563, 1522, 1483, 1456, 1406, 1386, 1359, 1328, 1117, 1041, 1028, 1013, 871, 824, 776, 750, 705, 683, 606, 505 cm−1; HRMS (ESI+) m/z calcd. for C12H13N2OS+ [M + H]+ 233.0743, found 233.0741, ∆ = −0.2 mDa.
3.3. Crystallography
Orange-coloured crystal blocks from the titled compound were obtained through recrystallization from iso-propanol. A suitable crystal with an appropriate size (0.25 × 0.2 × 0.1 mm3) was mounted on a nylon loop using cryoprotective Paratone oil. Diffraction data were collected on a Bruker D8 Venture diffractometer equipped with a IµS micro-focus sealed X-ray source (MoKα radiation, λ = 0.71073 Å) and a PHOTON II CPAD detector. Diffraction data were processed in the APEX4 software package [68]; the peaks were integrated with the Bruker SAINT software (ver. 2016/2) [69] using a narrow-frame algorithm. The intensities were scaled and the data were corrected for absorption effects using the multi-scan method (SADABS) [69]. The structure was solved with the intrinsic phasing method and refined with the full-matrix least-squares method on F2 (ShelxT and ShelxL program packages [70,71]) using OLEX—ver. 1.5 software [72]. All non-hydrogen atoms were located successfully from the Fourier map and were refined anisotropically. The hydroxyl hydrogen atom (H1) was located from the Fourier map and refined isotropically. The remaining hydrogen atoms were placed on calculated positions riding on the parent carbon atoms using the following scheme: Ueq = 1.2 for C-Haromatic = 0.93 Å and C-Hmethyl = 0.96 Å. ORTEP-3v2 software [73] was used to illustrate the molecule present in the asymmetric unit. A three-dimensional packing visualization of the molecules was made using CCDC Mercury [74]. The most important data collection and crystallographic refinement parameters for 3-methyl-1-phenyl-4-thioacetylpyrazol-5-one are given in Table S1. Complete crystallographic data for the reported structure have been deposited in the CIF format with the Cambridge Crystallographic Data Center as 2239465. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, deposited on 2 February 2023 (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +441223336033; E-mail: depos-it@ccdc.cam.ac.uk).
4. Conclusions
3-Methyl-1-phenyl-4-thioacetylpyrazol-5-one was obtained in excellent yield through the thionation of the corresponding acylpyrazolone by using Lawesson’s reagent. It was found that only acetyl oxygen was replaced by sulphur regardless of the reagent’s proportions. The product was isolated with column chromatography and characterized with 1D and 2D NMR, IR, and HRMS spectra. The single-crystal XRD revealed that 3-methyl-1-phenyl-4-thioacetylpyrazol-2-one crystallized in the monoclinic C2/c space group.
Supplementary Materials
13C, HSQC and HMBC NMR, ATR IR and ESI HR-MS spectra, Tables S1 and S2, CIF and checkcif report for the title compound.
Author Contributions
The synthetic experiments and NMR analyses were carried out by V.K. The ESI HR-MS spectrum was conducted by Z.P. The single-crystal XRD was performed by R.R. and B.S. All authors contributed to the discussion of the results and to writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The financial support by the Bulgarian National Science Fund, project KP 06-H69/5 from 2022, is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. On the Review of the List of Critical Raw Materials for the EU and the Implementation of the Raw Materials Initiative. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52014DC0297&from=EN (accessed on 26 May 2014).
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and recycling of by-products in the steel sector: Recent achievements paving the way to circular economy and industrial symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef]
- Iluţiu-Varvara, D.-A.; Aciu, C. Metallurgical wastes as resources for sustainability of the steel industry. Sustainability 2022, 14, 5488. [Google Scholar] [CrossRef]
- Jadhav, U.U.; Hocheng, H. A review of recovery of metals from industrial waste. J. Achiev. Mater. Manuf. 2012, 54, 159–167. [Google Scholar]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R. Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 2015, 5, 1–60. [Google Scholar] [CrossRef]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.-H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Sunder, G.S.S.; Adhikari, S.; Rohanifar, A.; Poudel, A.; Kirchhoff, J.R. Evolution of environmentally friendly strategies for metal extraction. Separations 2020, 7, 4. [Google Scholar] [CrossRef]
- Rasheed, M.Z.; Nam, S.-W.; Cho, J.-Y.; Park, K.-T.; Kim, B.-S.; Kim, T.-S. Review of the liquid metal extraction process for the recovery of Nd and Dy from permanent magnets. Metall. Mater. Trans. B 2021, 52, 1213–1227. [Google Scholar] [CrossRef]
- Qasem, H.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Vaughan, J.; Southam, G.; der Ent, A.; Nkrumah, P.N.; Ma, X.; Parbhakar-Fox, A. Review on metal extraction technologies suitable for critical metal recovery from mining and processing wastes. Miner. Eng. 2022, 182, 107537. [Google Scholar] [CrossRef]
- Bu, X.; Danstan, J.K.; Hassanzadeh, A.; Vakylabad, A.B.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching—An overview. Miner. Process. Extr. Metall. Rev. 2022, in press. [Google Scholar] [CrossRef]
- Kaye, P.T. Designer ligands: The search for metal ion selectivity. S. Afr. J. Sci. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.J.; Biros, S.M. Supramolecular ligands for the extraction of lanthanide and actinide ions. Org. Chem. Front. 2019, 6, 2067–2094. [Google Scholar] [CrossRef]
- Yudaev, P.; Chistyakov, E. Chelating extractants for metals. Metals 2022, 12, 1275. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarjadi, M.S.; Guerin, S.; Sarkar, S.M. Poly(amidoxime) resins for efficient and eco-friendly metal extraction. ACS Appl. Polym. Mater. 2022, 4, 2216–2232. [Google Scholar] [CrossRef]
- Boukayouht, K.; Bazzi, L.; El Hankari, S. Sustainable synthesis of metal-organic frameworks and their derived materials from organic and inorganic wastes. Coord. Chem. Rev. 2023, 478, 214986. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Drozdov, A. β-Diketones and related ligands. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; Volume 1, Chapter 1.6; pp. 97–115. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord. Chem. Rev. 2009, 253, 1099–1201. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Dukov, I. The interaction of extractants during synergistic solvent extraction of metals. Is it an important reaction? RSC Adv. 2016, 6, 81250–81265. [Google Scholar] [CrossRef]
- Atanassova, M. Thenoyltrifluoroacetone: Preferable molecule for solvent extraction of metals—Ancient twists to new approaches. Separations 2022, 9, 154. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Edilova, Y.O.; Burgart, Y.V.; Saloutin, V.I. Fluorinated 1,2,4-triketone analogs: New prospects for heterocyclic and coordination chemistry. Russ. Chem. Bull. 2022, 71, 1321–1341. [Google Scholar] [CrossRef]
- Saloutin, V.I.; Edilova, Y.O.; Kudyakova, Y.S.; Burgart, Y.V.; Bazhin, D.N. Heterometallic molecular architectures based on fluorinated β-diketone ligands. Molecules 2022, 27, 7894. [Google Scholar] [CrossRef] [PubMed]
- Zolotov, Y.A.; Kuzmin, N.M. Extraction of Metals by Acylpyrazolones; Nauka: Moscow, Russia, 1977. [Google Scholar]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone ligands: Synthesis, structures, metal coordination chemistry and applications. Coord. Chem. Rev. 2005, 249, 2909–2945. [Google Scholar] [CrossRef]
- Binnemans, K. Rare-earth beta-diketonates. In Handbook on the Physics and Chemistry of Rare Earths; Gschneider, K.A., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier B. V.: Burlington, NJ, USA, 2005; Volume 35, Chapter 225; pp. 107–272. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, R.; Pettinari, C. Recent advances in acylpyrazolone metal complexes and their potential applications. Coord. Chem. Rev. 2015, 303, 1–31. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Di Nicola, C.; Tombesi, A.; Pettinari, R. Coordination chemistry of pyrazolone-based ligands and applications of their metal complexes. Coord. Chem. Rev. 2019, 401, 213069. [Google Scholar] [CrossRef]
- Bao, X.; Wang, X.; Tian, J.-M.; Ye, X.; Wang, B.; Wang, H. Recent advances in the applications of pyrazolone derivatives in enantioselective synthesis. Org. Biomol. Chem. 2022, 20, 2370–2386. [Google Scholar] [CrossRef]
- Kennedy, B.P.; Leve, A.B.P. Studies of the metal-sulfur bond. Complexes of the pyridine thiols. Can. J. Chem. 1972, 50, 3488–3507. [Google Scholar] [CrossRef]
- Stiefel, E.I. Transition Metal Sulfur Chemistry: Biological and Industrial Significance and Key Trends; Transition Metal Sulfur Chemistry; Stiefel, E.I., Matsumoto, K., Eds.; ACS Symposium Series: Washington, DC, USA, 1996; Volume 356, pp. 2–38. [Google Scholar] [CrossRef]
- Petz, W. 40 Years of transition-metal thiocarbonyl chemistry and the related CSe and CTe compounds. Coord. Chem. Rev. 2008, 252, 1689–1733. [Google Scholar] [CrossRef]
- Schenk, W.A. The coordination chemistry of small sulfur-containing molecules: A personal perspective. Dalton Trans. 2011, 40, 1209–1219. [Google Scholar] [CrossRef]
- Wachter, J. Synthesis, structure and reactivity of sulfur-rich cyclopentadienyl-transition metal complexes: Sulfur chemistry from an organometallic point of view. Angew. Chem. Int. Ed. 1989, 28, 1613–1626. [Google Scholar] [CrossRef]
- Mensforth, E.J.; Hill, M.R.; Batten, S.R. Coordination polymers of sulphur-donor ligands. Inorg. Chim. Acta 2013, 403, 9–24. [Google Scholar] [CrossRef]
- Paradiso, V.; Capaccio, V.; Lamparelli, D.H.; Capacchione, C. Metal complexes bearing sulfur-containing ligands as catalysts in the reaction of CO2 with epoxides. Catalysts 2020, 10, 825. [Google Scholar] [CrossRef]
- Hou, J.-T.; Kwon, N.; Wang, S.; Wang, B.; He, X.; Yoon, J.; Shen, J. Sulfur-based fluorescent probes for HOCl: Mechanisms, design, and applications. Coord. Chem. Rev. 2022, 450, 214232. [Google Scholar] [CrossRef]
- Bingham, N.M.; Abousalman-Rezvani, Z.; Collins, K.; Roth, P.J. Thiocarbonyl chemistry in polymer science. Polym. Chem. 2022, 13, 2880–2901. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.-Q. A Review of heteroatom doped materials for advanced lithium–sulfur batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, S.-L.; Zhong, Y.-H.; Hu, J.; Chung, L.-H.; He, J. Conductive MOFs based on thiol-functionalized linkers: Challenges, opportunities, and recent advances. Coord. Chem. Rev. 2022, 450, 214235. [Google Scholar] [CrossRef]
- Hao, H.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.; Mitlin, D. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal–sulfur and selenium batteries. Chem. Rev. 2022, 122, 8053–8125. [Google Scholar] [CrossRef]
- Yoshinari, N.; Kuwamura, N.; Kojima, T.; Konno, T. Development of coordination chemistry with thiol-containing amino acids. Coord. Chem. Rev. 2023, 474, 214857. [Google Scholar] [CrossRef]
- Petrova, M.; Kurteva, V. Synergistic efficiency of 2-[(1-aza-15-crown-5)-1-ylmethyl)]-4-(phenyldiazenyl)-naphtalen-1-ol in the Liquid Extraction of Light Lanthanoid(III) ions with 4-benzoyl-3-phenyl-5-isoxazolone. The role of aza-crown and azo-dye fragments on the extraction ability. J. Chem. Eng. Data 2014, 59, 1295–1303. [Google Scholar] [CrossRef]
- Petrova, A.A.; Angelova, S.M.; Nikolchina, I.A.; Russev, R.I.; Kurteva, V.B.; Shivachev, B.L.; Petrova, R.N. Novel 13-membered cyclic dioxatetraaza scaffolds–synthesis, solution and solid state characterization. Bulg. Chem. Commun. 2015, 47, 208–220. [Google Scholar]
- Atanassova, M.; Kurteva, V. Synergism as a phenomenon in solvent extraction of 4f-elements with calixarenes. RSC Adv. 2016, 6, 11303–11324. [Google Scholar] [CrossRef]
- Kurteva, V.; Lubenov, L.; Petrova, M. Selective C-acylation of 3-methyl-1-phenyl-pyrazol-5-one. In Comprehensive Organic Chemistry Experiments for the Laboratory Classroom (COCELC); Afonso, C.A.M., Candeias, N.R., Pereira Simão, D., Trindade, A.F., Coelho, J.A.S., Tan, B., Franzén, R., Eds.; RSC Publishing: Cambridge, UK, 2017; Chapter 2.2.1; pp. 107–111. [Google Scholar]
- Petrova, M.A.; Todorova, S.E.; Kurteva, V.B.; Todorova, N.I. Insights into the synergistic selectivity of 4f-ions implementing 4-acyl-5-pyrazolone and two new unsymmetrical NH-urea containing ring molecules in an ionic liquid. Sep. Purif. Technol. 2018, 204, 328–335. [Google Scholar] [CrossRef]
- Kurteva, V.B.; Lubenov, L.A.; Shivachev, B.L.; Nikolova, R.P.; Fromm, K.M. Betti bases from 4-(3-pyridazo)-1-naphthol: Synthesis, coordination behaviour and unusual substitution reactions. ChemistrySelect 2018, 3, 12017–12021. [Google Scholar] [CrossRef]
- Todorova, S.E.; Rusew, R.I.; Petkova, Z.S.; Shivachev, B.L.; Nikolova, R.P.; Kurteva, V.B. Acylpyrazolones possessing heterocyclic moiety in the acyl fragment: Intramolecular vs. intermolecular zwitterionic structure. New J. Chem. 2022, 46, 1080–1086. [Google Scholar] [CrossRef]
- Cooper, N.J. Thioaldehydes and thioketones. In Comprehensive Organic Functional Group Transformations II; Katritzky, A.R., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2005; Volume 3, Chapter 3.08; pp. 355–396. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, X. Transfer of sulfur: From simple to diverse. Chem. Asian J. 2013, 8, 2546–2563. [Google Scholar] [CrossRef]
- Murai, T. The construction and application of C=S bonds. Top. Curr. Chem. 2018, 376, 31. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Mondal, B.; Saha, J. Recent developments on the synthesis of various sulfur-containing heterocycles via [3+2]-and [4+2]-cycloaddition reactions with thiocarbonyls. Asian J. Org. Chem. 2020, 9, 1466–1477. [Google Scholar] [CrossRef]
- Jesberger, M.; Davis, T.P.; Barner, L. Applications of Lawesson’s reagent in organic and organometallic syntheses. Synthesis 2003, 1929–1958. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Praliyev, K.D.; Gut′Yar, V.G.; Baitursynova, G.P. Modification of organic compounds with Lawesson’s reagent. Russ. J. Org. Chem. 2015, 51, 148–160. [Google Scholar] [CrossRef]
- Larik, F.A.; Saeed, A.; Muqadar, U.; Channar, P.A. Application of Lawesson’s reagent in the synthesis of sulfur-containing medicinally significant natural alkaloids. J. Sulfur Chem. 2017, 38, 206–227. [Google Scholar] [CrossRef]
- Bergman, J. Comparison of two reagents for thionations. Synthesis 2018, 50, 2323–2328. [Google Scholar] [CrossRef]
- Gayen, K.S.; Chatterjee, N. Diversity of Lawesson’s reagent: Advances and scope. Asian J. Org. Chem. 2020, 9, 508–528. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdulmalek, E. A focused review of synthetic applications of Lawesson’s reagent in organic synthesis. Molecules 2021, 26, 6937. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, X.; Xiong, Y.; Yin, G.; Liu, L.; Wang, Z. Lawesson’s reagent-mediated deoxygenation reactions. ChemistrySelect 2022, 7, e202201748. [Google Scholar] [CrossRef]
- Kittikool, T.; Yotphan, S. Metal-free direct C–H thiolation and thiocyanation of pyrazolones. Eur. J. Org. Chem. 2020, 2020, 961–970. [Google Scholar] [CrossRef]
- Langler, R.F. Synthesis and structure of 3-methyl-1-phenyl-4-sulfhydrylbenzylidene-5-thiopyrazolone. Can. J. Chem. 1971, 49, 481–484. [Google Scholar] [CrossRef]
- Sayed, G.H.; Shiba, S.A.; Radwan, A.; Mohamed, S.M.; Khalil, M. Synthesis and reactions of some 6-aryl and 2,6-diaryl-4-(4′-antipyrinyl)-2,3,4,5-tetrahydropyridazin-3-ones and screening for their antibacterial activities. Chin. J. Chem. 1992, 10, 475–480. [Google Scholar] [CrossRef]
- Tagawa, Y.; Minami, S.; Yoshida, T.; Tanaka, K.; Sato, S.; Goto, Y.; Yamagata, K. Preparation and antibacterial activity of 3-methyl-1-p-substituted phenylpyrazole-5-thiol. Arch. Pharm. Pharm. Med. Chem. 2002, 2, 99–103. [Google Scholar] [CrossRef]
- Müller, C.; Ma, B.N.; Gust, R.; Bernkop-Schnürch, A. Thiopyrazole preactivated chitosan: Combining mucoadhesion and drug delivery. Acta Biomater. 2013, 9, 6585–6593. [Google Scholar] [CrossRef]
- Callaghan, P.D.; Elliott, A.J.; Gandhi, S.S.; Gibson, M.S.; Mastalerr, H.; Vukov, D.J. Acylation of N′-arylbenzothiohydrazides and of their N′-acyl-derivatives; 2-acylalkylidene-3-aryl-5-phenyl-2H-1,3,4-thiadiazolenes and related compounds. J. Chem. Soc. Perkin Trans. 1981, 1, 2948–2951. [Google Scholar] [CrossRef]
- Bruker. APEX 3. In Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Bruker, A. Saint and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Section C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).