Abstract
Organocatalyzed synthesis of tert-butyl 2-amino-3-cyano-5-oxo-4-phenyl-5,7-dihydropyrano[2,3-c]pyrrole-6(4H)-carboxylate, prepared from Boc-tetramic acid and benzylidenemalononitrile, is disclosed. Two bifunctional noncovalent organocatalysts were employed, yielding the product as a racemic mixture in both cases. The structure of the new synthesized compound was confirmed by high resolution mass-spectrometry, 1H- and 13C-NMR, HSQC, and IR spectroscopy.
1. Introduction
Tetramic acids (pyrrolidine-2,4-dione derivatives) represent an important, structurally diverse group of naturally occurring compounds isolated from various terrestrial and marine species, such as sponges, bacteria, and fungi. Both naturally occurring tetramic acids and their synthetic analogues attracted considerable attention due to their diverse and promising bioactivities [1,2,3,4,5].
Different synthetic protocols have been applied for the construction of the tetramic acid scaffold [6]. C-3/5 unsubstituted tetramic acids are conveniently prepared in two steps via the C-acylation reaction of Meldrum’s acid with activated, N-protected α-amino acid followed by thermal decomposition of the meldrumate intermediate [7,8,9,10,11]. C-3/5 unsubstituted tetramic acids represent viable building blocks for the construction of libraries of more complex tetramic acid derivatives with potentially beneficial biological activities.
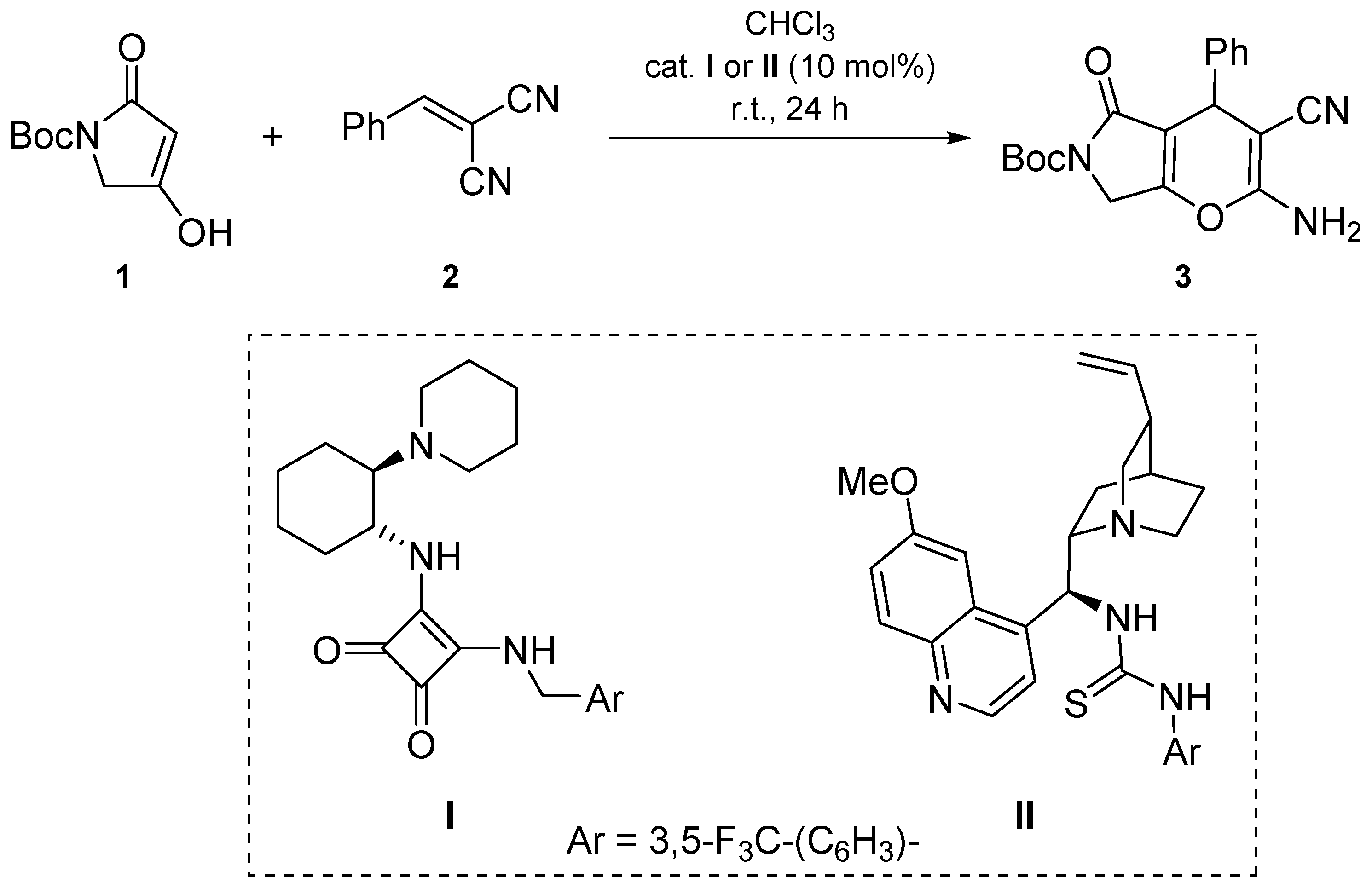
Annulation of either acyclic or (hetero)cyclic Michael acceptors with malononitrile is a convenient approach for the stereoselective synthesis of amino-cyano-substituted 4H-pyran heterocycles and their fused analogues [12,13,14,15]. In continuation of our research on the implementation of pyrrolone derivatives in organocatalyzed asymmetric transformations, [16,17,18,19,20] we herein report the application of N-Boc substituted tetramic acids 1 in the construction of dihydropyrano[2,3-c]pyrrole heterocycle 3 via organocatalyzed conjugative addition to benzylidenemalononitrile (2) followed by cyclization.
2. Results and Discussion
Recently, we reported the application of C-3/5 unsubstituted tetramic and tetronic acid derivatives in the organocatalyzed alkylation with trans-β-nitrostyrene derivatives and their aliphatic analogues [21]. Among the screened catalysts, the best results were obtained with cyclohexane-1,2-diamine derived squaramide catalyst I [22], yielding 1,4-addition products with enantioselectivities up to 94% ee. In continuation of our studies on the implementation of tetramic acid derivatives in organocatalyzed functionalizations, benzylidenemalononitrile (2) was applied as the Michael acceptor in the reaction with Boc-tetramic acid 1 [21] using I as the catalyst (Scheme 1). Dihydropyrano[2,3-c]pyrrole 3 was formed in 36% yield, presumably via the initial Michael addition, followed by 6-exo-dig enolate-to-nitrile cyclisation, and final tautomerization of the imine into enamine.
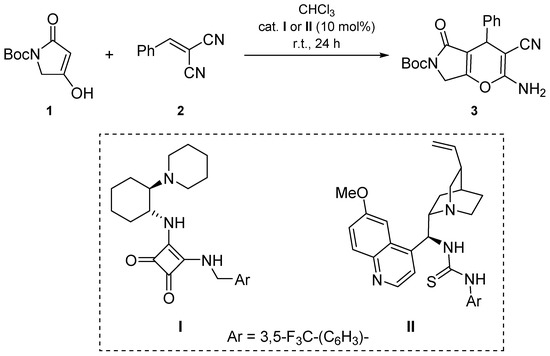
Scheme 1.
Organocatalyzed synthesis of dihydropyrano[2,3-c]pyrrole 3.
Unexpectedly, the product 3 was formed as a racemate, as confirmed by HPLC analysis. Repeating the reaction with quinuclidine-derived thiourea catalyst II [23] furnished dihydropyrano[2,3-c]pyrrole 3 in 32% yield as a racemate as well. Other organocatalysts have not been employed. Enantiomerically enriched dihydropyrano[3,2-b]pyrrole analogues have been successfully prepared via organocatalyzed annulation of arylidene-Δ2-pyrrolin-4-ones with malononitrile [20]. For the synthesis of racemic dihydropyrano[2,3-c]pyrroles, an achiral organocatalyst, 3-((3,5-bis(trifluoromethyl)benzyl)amino)-4-((2-(dimethylamino)ethyl)amino)cyclobut-3-ene-1,2-dione, could be used [24].
The structure of compound 3 was confirmed by spectroscopic methods (1H- and 13C-NMR, HSQC, IR, and high-resolution mass spectrometry (HRMS)). The racemic nature of product 3 was confirmed by HPLC analysis.
In conclusion, dihydropyrano[2,3-c]pyrrole 3 was successfully synthesized as a racemic mixture from Boc-tetramic acid 1 and benzylidenemalononitrile (2). In view of the commercial availability of arylidene-malononitrile derivatives and straightforward preparation of tetramic acid derivatives from amino acids [21], a library of (racemic) dihydropyrano[2,3-c]pyrroles can easily be prepared and studied further.
3. Materials and Methods
Solvents for extractions and chromatography were of technical grade and were distilled prior to use. Extracts were dried over technical grade anhydrous Na2SO4. Melting points were determined on a Kofler micro hot stage and on SRS OptiMelt MPA100—Automated Melting Point System (Stanford Research Systems, Sunnyvale, CA, USA). The NMR spectra were obtained on a Bruker UltraShield 500 plus (Bruker, Billerica, MA, USA) at 500 MHz for 1H and 126 MHz for 13C nucleus, using DMSO-d6 and CDCl3 with TMS as the internal standard, as solvents. Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA), IR spectra on a Perkin-Elmer Spectrum BX FTIR spectrophotometer (PerkinElmer, Waltham, MA, USA). Column chromatography (CC) was performed on silica gel (Silica gel 60, particle size: 0.035–0.070 mm (Sigma-Aldrich, St. Louis, MI, USA)). HPLC analyses were performed on an Agilent 1260 Infinity LC (Agilent Technologies, Santa Clara, CA, USA) using CHIRALPAK AD-H (0.46 cm ø × 25 cm) as the chiral column (Chiral Technologies, Inc., West Chester, PA, USA). All the commercially available chemicals used were purchased from Sigma-Aldrich (St. Louis, MI, USA).
Organocatalysts I [22], II [23] and tetramic acid, tert-butyl 4-hydroxy-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxylate (1) [21], were prepared following the literature procedures.
Synthesis of tert-Butyl 2-Amino-3-cyano-5-oxo-4-phenyl-5,7-dihydropyrano[2,3-c]pyrrole-6(4H)-carboxylate (3)
To a mixture of tert-butyl 2,4-dioxopyrrolidine-1-carboxylate (1) (49.1 mg, ω = 81% (the purity of tetramic acid 1 was determined by 1H-NMR analysis using 1,3,5-trimethoxybenzene as the internal standard [21]), 0.20 mmol), benzylidenemalononitrile (2) (46.3 mg, 0.30 mmol), and organocatalyst I (10.1 mg, 0.02 mmol) or II (10.1 mg, 0.02 mmol), chloroform (1 mL) was added. The reaction mixture under argon was left to stir at 25 °C for 24 h. Volatile components were evaporated in vacuo. The residue was purified by column chromatography (Silica Gel 60; 1. EtOAc/petroleum ether = 1:2 to remove the nonpolar impurities, 2. EtOAc to elute the product 3). Fractions containing product 3 were combined and volatile components evaporated in vacuo. Product 3 formed as a racemic mixture. Yield: 25.4 mg (0.0720 mmol, 36%) of white solid; mp 166–169 °C. EI-HRMS: m/z = 298.0821 (M-tBu)H+; C15H12N3O4 requires: m/z = 298.0822 (M-tBu)H+; νmax 3479, 3310, 2978, 2198, 1761, 1703, 1630, 1577, 1455, 1419, 1383, 1343, 1310, 1265, 1252, 1199, 1148, 1093, 983, 946, 915, 841, 821, 804, 773, 735, 700, 654, 623 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 1.43 (s, 9H, tBu); 4.24 (s, 1H); 4.42 (dd, J = 1.9; 17.7 Hz, 1H); 4.51 (dd, J = 1.0; 17.7 Hz, 1H); 7.22–7.29 (m, 5H); 7.31–7.36 (m, 2H). 13C-NMR (126 MHz, DMSO-d6): δ 27.70, 35.23, 46.31, 57.80, 81.87, 108.51, 119.47, 127.13, 127.83, 128.40, 142.11, 148.95, 159.20, 161.81, 164.81. HPLC: Chiralpak AD-H, n-Hexane/i-PrOH = 90:10, flow rate 1.0 mL/min, λ = 230 nm. Enantiomers: tR = 13.5 min; 25.9 min—racemate (supplementary materials).
Supplementary Materials
Synthesis and characterization data; HPLC data; Copies of 1H- and 13C-NMR spectra; Copies of HRMS reports; IR spectra.
Author Contributions
Conceptualization, L.C., U.G., J.S. and B.Š.; methodology, L.C. and U.G.; software, L.C., U.G., J.S. and B.Š.; validation, L.C., U.G., J.S., F.P. and B.Š.; formal analysis, U.G., M.H. and L.C.; investigation, M.H. and U.G.; resources, L.C., U.G. and J.S.; data curation, L.C., U.G., J.S. and B.Š.; writing—original draft preparation, U.G., J.S. and B.Š.; writing—review and editing, L.C., U.G., J.S., F.P. and B.Š.; visualization, L.C., U.G., B.Š. and J.S.; supervision, U.G.; project administration, U.G. and J.S.; funding acquisition, U.G. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency through grant P1-0179.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the EN-FIST Centre of Excellence, Dunajska 156, 1000 Ljubljana, Slovenia, for the use of their BX FTIR spectrophotometer and Agilent 1260 Infinity LC for the HPLC analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghisalberti, E.L. Bioactive tetramic acid metabolites. Stud. Nat. Prod. Chem. 2003, 28, 109–163. [Google Scholar] [CrossRef]
- Royles, B.J.L. Naturally occurring tetramic acids: Structure, isolation, and synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Berek-Nagy, P.J.; Csíkos, S.; Tóth, G.; Bősze, S.; Horváth, L.B.; Darcsi, A.; Knapp, D.G.; Kovács, G.M.; Boldizsár, I. The grass root endophytic fungus Flavomyces fulophazii: An abundant source of tetramic acid and chlorinated azaphilone derivatives. Phytochemistry 2021, 190, 112851. [Google Scholar] [CrossRef]
- Mo, X.; Li, Q.; Ju, J. Naturally occurring tetramic acid products: Isolation, structure elucidation and biological activity. RSC Adv. 2014, 4, 50566–50593. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, S.; Li, J.; Liu, L. The biological and chemical diversity of tetramic acid compounds from marine-derived microorganisms. Mar. Drugs 2020, 18, 114. [Google Scholar] [CrossRef]
- Matiadis, D. Metal-catalyzed and metal-mediated approaches to the synthesis and functionalization of tetramic acids. Catalysts 2019, 9, 50. [Google Scholar] [CrossRef]
- Hamilakis, S.; Kontonassios, D.; Sandris, C. Acylaminoacetyl derivatives of active methylene compounds. J. Heterocycl. Chem. 1996, 33, 825–829. [Google Scholar] [CrossRef]
- Jouin, P.; Castro, B.; Nisato, D. Stereospecific synthesis of N-protected statine and its analogues via chiral tetramic acid. J. Chem. Soc. Perkin Trans. 1 1987, 1177–1182. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Moloney, M.G. Tetramic acids as scaffolds: Synthesis, tautomeric and antibacterial behaviour. Synlett 2009, 2009, 2487–2491. [Google Scholar] [CrossRef]
- Liu, Z.; Ruan, X.; Huang, X. A facile solid phase synthesis of tetramic acid. Bioorg. Med. Chem. Lett. 2003, 13, 2505–2507. [Google Scholar] [CrossRef]
- Pirc, S.; Bevk, D.; Jakše, R.; Rečnik, S.; Golič, L.; Golobič, A.; Meden, A.; Stanovnik, B.; Svete, J. Synthesis of N-substituted 3-aminomethylidenetetramic acids. Synthesis 2005, 17, 2969–2988. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Lou, C.-L.; Wang, J.-J.; Chen, C.-X.; Yan, M. Organocatalytic conjugate addition of alononitrile to conformationally restricted dienones. J. Org. Chem. 2011, 76, 3797–3804. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Wang, W.-J.; Yin, X.-G.; Zhang, X.-J.; Yan, M. Enantioselective synthesis of 2-amino-4H-pyrans via the organocatalytic cascade reaction of malononitrile and α-substituted chalcones. Tetrahedron Asymmetry 2012, 23, 461–467. [Google Scholar] [CrossRef]
- Zhao, S.-L.; Zheng, C.-W.; Zhao, G. Enantioselective synthesis of multifunctionalized 4H-pyran derivatives using bifunctional thiourea-tertiary amine catalysts. Tetrahedron Asymmetry 2009, 20, 1046–1051. [Google Scholar] [CrossRef]
- Wang, H.X.; Wu, L.L.; Wang, Y.M.; Zhou, Z.H. Organocatalyzed asymmetric tandem Michael-cyclization reaction of 4-benzylidene-3-methylpyrazol-5-ones and malononitrile: Stereocontrolled construction of pyrano[2,3-c]pyrazole scaffold. RSC Adv. 2015, 5, 42836–42842. [Google Scholar] [CrossRef]
- Ričko, S.; Meden, A.; Ivančič, A.; Perdih, A.; Štefane, B.; Svete, J.; Grošelj, U. Organocatalyzed deracemization of Δ2-pyrrolin-4-ones. Adv. Synth. Catal. 2017, 359, 2288–2296. [Google Scholar] [CrossRef]
- Ričko, S.; Meden, A.; Ciber, L.; Štefane, B.; Požgan, F.; Svete, J.; Grošelj, U. Construction of vicinal tetrasubstituted stereogenic centers via a mannich-type organocatalyzed addition of Δ2-pyrrolin-4-ones to isatin imines. Adv. Synth. Catal. 2018, 360, 1072–1076. [Google Scholar] [CrossRef]
- Grošelj, U.; Ciber, L.; Gnidovec, J.; Testen, Ž.; Požgan, F.; Štefane, B.; Tavčar, G.; Svete, J.; Ričko, S. Synthesis of spiro-Δ2-pyrrolin-4-one pseudo enantiomers via an organocatalyzed sulfa-Michael/aldol domino sequence. Adv. Synth. Catal. 2019, 361, 5118–5126. [Google Scholar] [CrossRef]
- Ričko, S.; Testen, Ž.; Ciber, L.; Požgan, F.; Štefane, B.; Brodnik, H.; Svete, J.; Grošelj, U. Double spirocyclization of arylidene-Δ2-pyrrolin-4-ones with 3-isothiocyanato oxindoles. Catalysts 2020, 10, 1211. [Google Scholar] [CrossRef]
- Ciber, L.; Ričko, S.; Gregorc, J.; Požgan, F.; Svete, J.; Brodnik, H.; Štefane, B.; Grošelj, U. Mechanistic insights into annulation of arylidene-Δ2-pyrrolin-4-ones by cinchona squaramide-based organocatalysts. Adv. Synth. Catal. 2022, 364, 980–993. [Google Scholar] [CrossRef]
- Ciber, L.; Gorenc, A.; Hozjan, M.; Požgan, F.; Svete, J.; Brodnik, H.; Štefane, B.; Grošelj, U. Enantioselective organocatalyzed functionalization of tetramic and tetronic acids. Adv. Synth. Catal. 2022, 364, 3840–3855. [Google Scholar] [CrossRef]
- Baran, R.; Veverková, E.; Škvorcová, A.; Šebesta, R. Enantioselective Michael addition of 1,3-dicarbonyl compounds to a nitroalkene catalyzed by chiral squaramides—A key step in the synthesis of pregabalin. Org. Biomol. Chem. 2013, 11, 7705–7711. [Google Scholar] [CrossRef] [PubMed]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Ričko, S.; Svete, J.; Štefane, B.; Perdih, A.; Golobič, A.; Meden, A.; Grošelj, U. 1,3-diamine-derived bifunctional organocatalyst prepared from camphor. Adv. Synth. Catal. 2016, 358, 3786–3796. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).