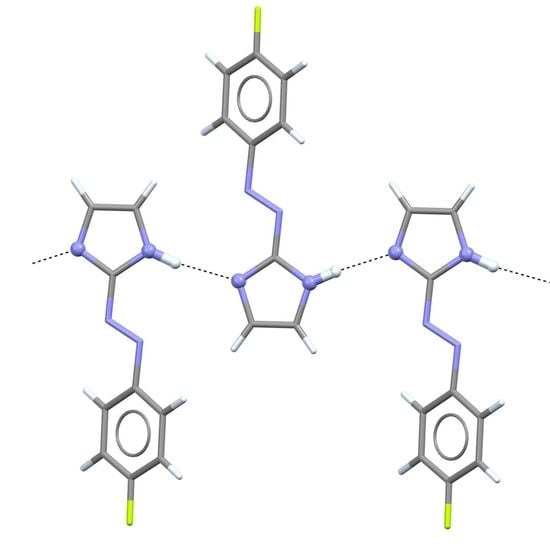
Crystal Structure of 2-[(1E)-2-(4-Fluorophenyl)diazenyl]-1H-imidazole
Abstract
:1. Introduction
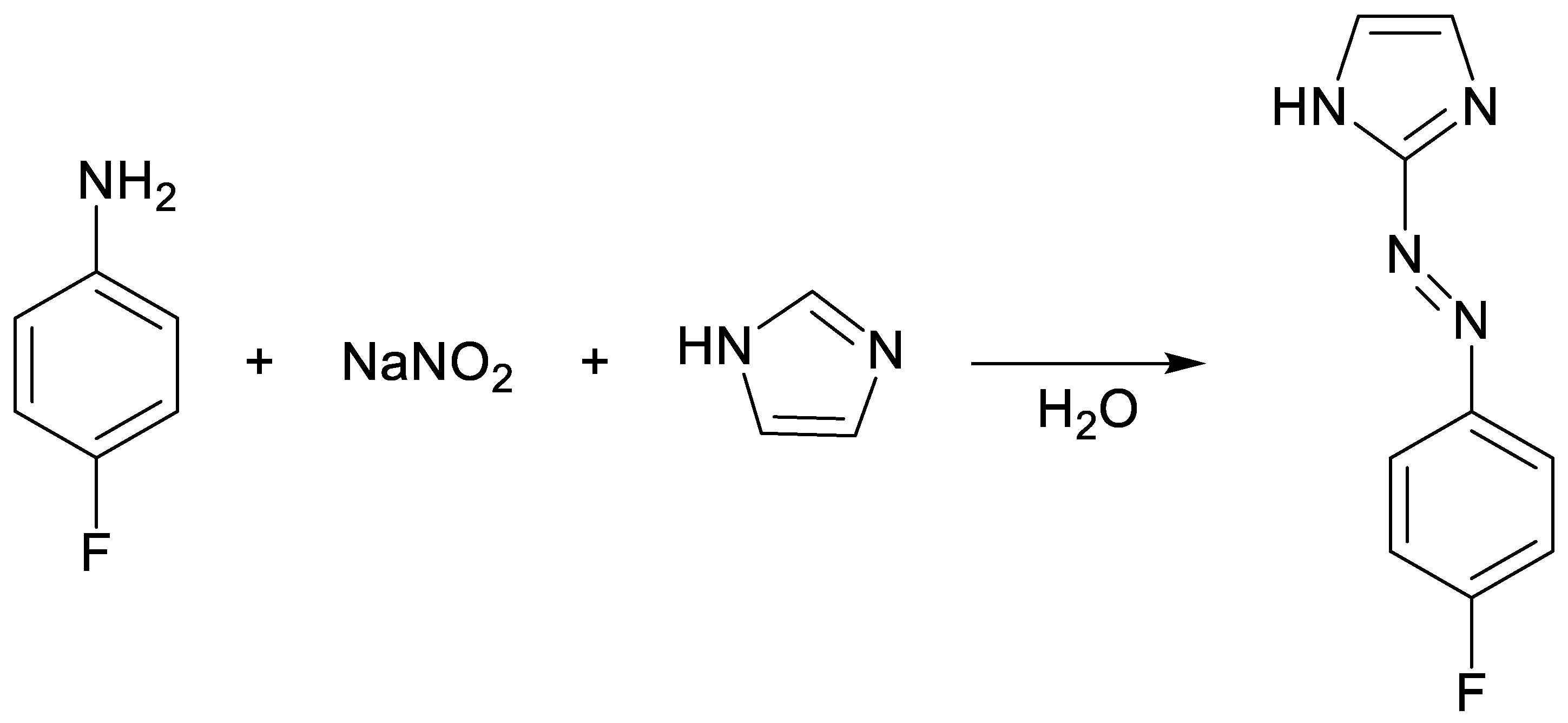
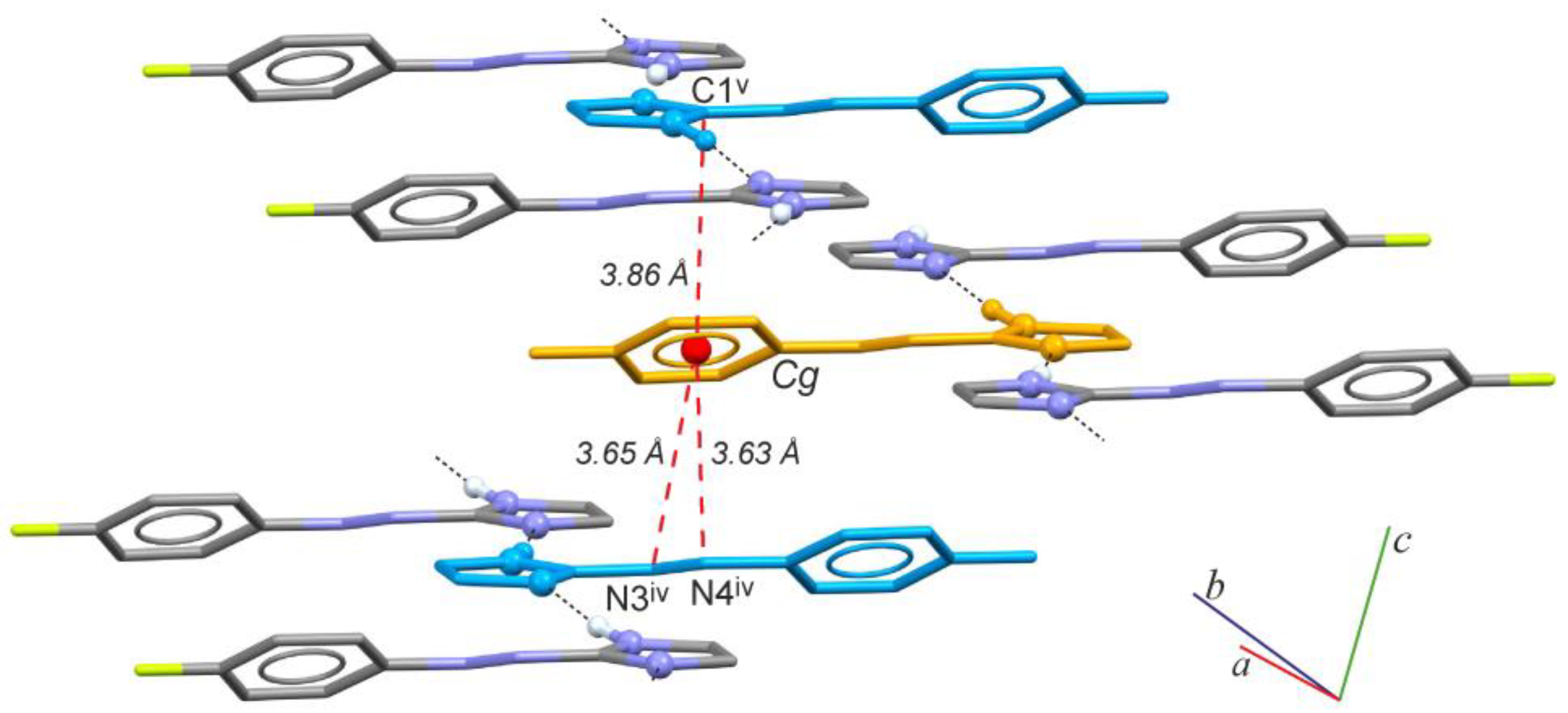
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuner, S.; Schwartz, H.; Kreutz, C.; Mueller, T.; Mayer, P.; Bonn, G.; Gelbrich, T.; Griesser, U.J.; Wurst, K.; Kahlenberg, V.; et al. 2-Arylimidazole revamped by quarternization or dimerization; another gain in functionality of an industrial dyestuff family by task-specific side-chain subsitutents. Heterocycles 2022, 105, 461–476. [Google Scholar]
- Bachmann, F.; Cremer, C.; Froehling, B.; Murphy, B.; Zhang, G.; Torgerson, P. Cationic Direct Dyes for Use in Hair Dying. WO Patent WO2016146813A1, 18 March 2016. [Google Scholar]
- Klingsberg, E.; Lewis, C.E. Quarternized N-Alkylated Arylazoimidazoles. U.S. Patent US3173907, 27 November 1959. [Google Scholar]
- Baumann, H.; Dehnert, J. New synthesis of diazastyryl dyes. Chimia 1961, 15, 163–168. [Google Scholar]
- Fun, H.-K.; Chinnakali, K.; Chen, X.-F.; Zhu, X.-H.; You, X.-Z. (1H-Imidazol-2-yl)phenyldiazene. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 9900025. [Google Scholar] [CrossRef]
- Bhunia, P.; Baruri, B.; Ray, U.; Sinha, C.; Das, S.; Cheng, J.; Lu, T.-H. Azoimidazole Complexes of Manganese(II)-thiocyanate. X-ray Structures of 2-(p-tolylazo)imidazole and Bis-[1-methyl-2-(p-tolylazo)imidazole]-Di-thiocyanato-manganese(II). Transition Met. Chem. 2006, 31, 310–315. [Google Scholar] [CrossRef]
- Pramanik, A.; Majumdar, S.; Das, G. Arylazoimidazoles assisted assembly of anion/anion–water through salt formation. CrystEngComm 2010, 12, 250–259. [Google Scholar] [CrossRef]
- Lin, C.; Yang, L.; Xu, M.; An, Q.; Xiang, Z.; Liu, X. Properties and applications of designable and photo/redox dual responsive surfactants with the new head group 2-arylazo-imidazolium. RSC Adv. 2016, 6, 51552–51561. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A-Fundam. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


| Empirical formula | C9H7FN4 |
| Formula weight | 190.19 |
| Temperature (K) | 183 |
| Wavelength (Å) | 0.71073 |
| Crystal system | Triclinic |
| Space group | |
| a (Å) | 7.0756(4) |
| b (Å) | 7.7929(5) |
| c (Å) | 8.8199(6) |
| α (°) | 70.801(2)° |
| β (°) | 74.9153(19)° |
| γ (°) | 83.689(2)° |
| Unit cell volume (Å3) | 443.29(5) |
| Z/Z’ | 2/1 |
| Reflections collected/Rint | 10418/0.0405 |
| Data/restraints/parameters | 1632/2/136 |
| Goodness-of-fit on F2 | 1.036 |
| R1 [I > 2 σ(I)] | 0.0344 |
| wR2 (all data) | 0.0943 |
| Largest diff. peak and hole (e · Å−3) | 0.216 and −0.199 |
| CCDC no. | 2229643 |
| D–H···A | d(D–H) | d(H···A) | d(D···A) | D–H···A |
|---|---|---|---|---|
| N1–H1N···N1 i | 0.873(10) | 2.011(11) | 2.876(2) | 171(3) |
| N2–H2N···N2 ii | 0.870(10) | 1.944(10) | 2.810(2) | 174(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelbrich, T.; Wurst, K.; Mayer, P.; Schottenberger, H.; Nerdinger, S. Crystal Structure of 2-[(1E)-2-(4-Fluorophenyl)diazenyl]-1H-imidazole. Molbank 2023, 2023, M1571. https://doi.org/10.3390/M1571
Gelbrich T, Wurst K, Mayer P, Schottenberger H, Nerdinger S. Crystal Structure of 2-[(1E)-2-(4-Fluorophenyl)diazenyl]-1H-imidazole. Molbank. 2023; 2023(1):M1571. https://doi.org/10.3390/M1571
Chicago/Turabian StyleGelbrich, Thomas, Klaus Wurst, Paul Mayer, Herwig Schottenberger, and Sven Nerdinger. 2023. "Crystal Structure of 2-[(1E)-2-(4-Fluorophenyl)diazenyl]-1H-imidazole" Molbank 2023, no. 1: M1571. https://doi.org/10.3390/M1571
APA StyleGelbrich, T., Wurst, K., Mayer, P., Schottenberger, H., & Nerdinger, S. (2023). Crystal Structure of 2-[(1E)-2-(4-Fluorophenyl)diazenyl]-1H-imidazole. Molbank, 2023(1), M1571. https://doi.org/10.3390/M1571