Diverse Hydrogen-Bonded Structural Motifs in 1,4-Diazabicyclo[2.2.2]octane N,N’-Dioxide Salts with Oxoanions
Abstract
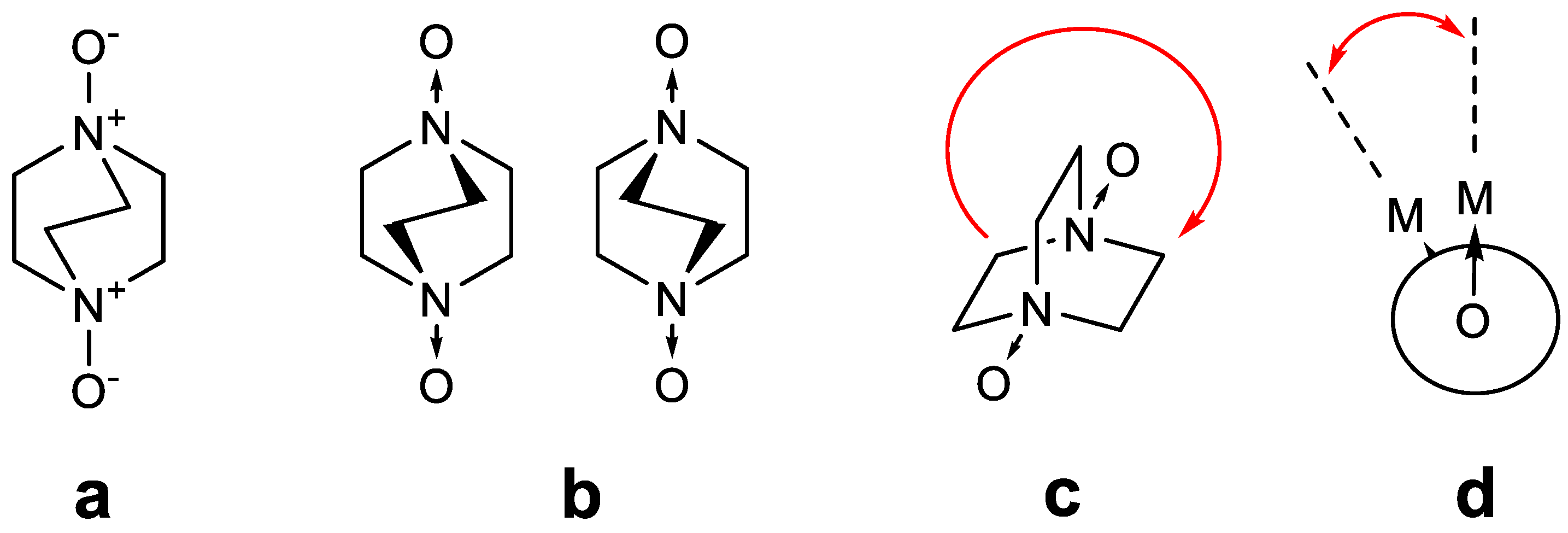
1. Introduction
2. Results and Discussion
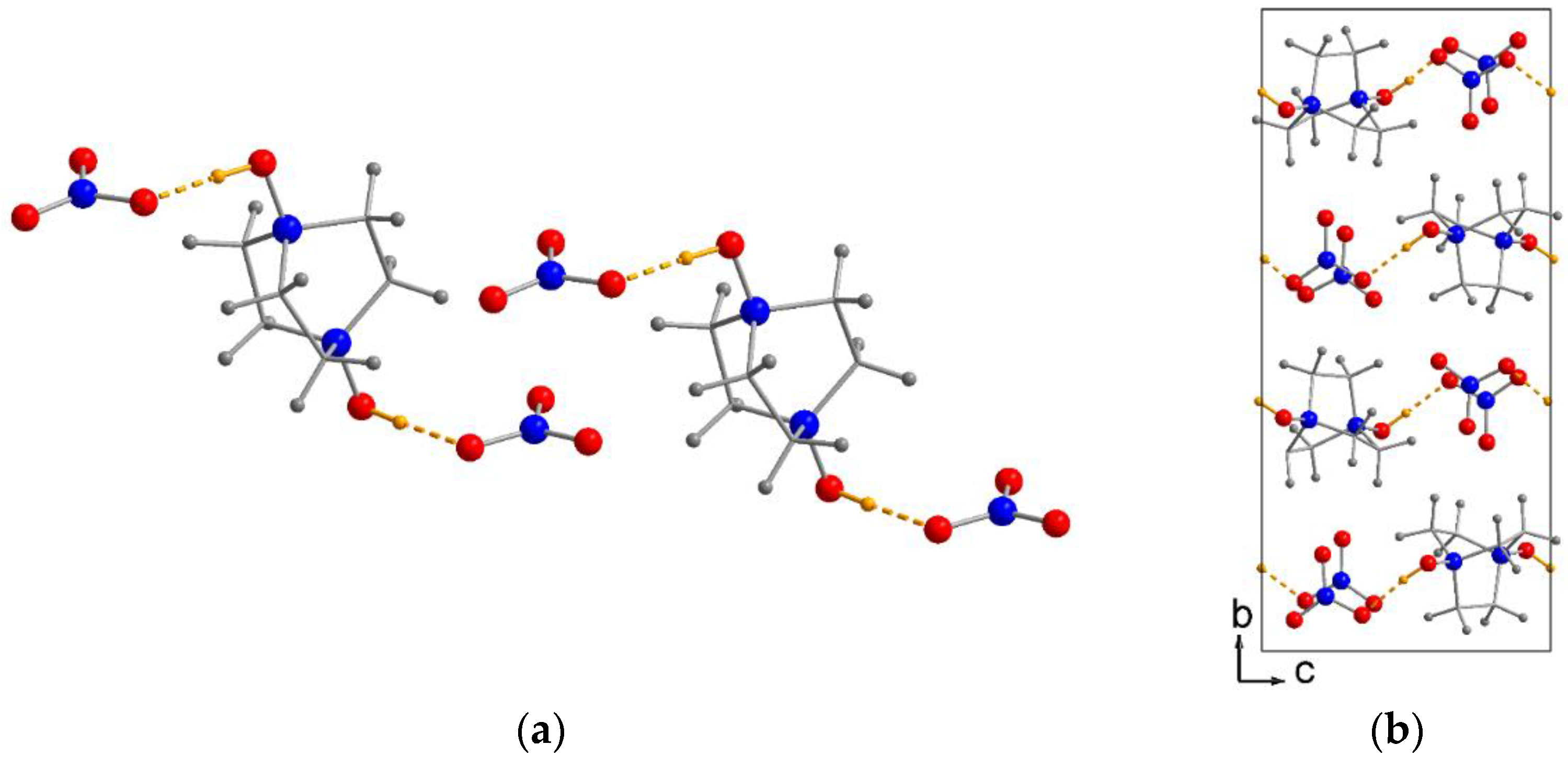
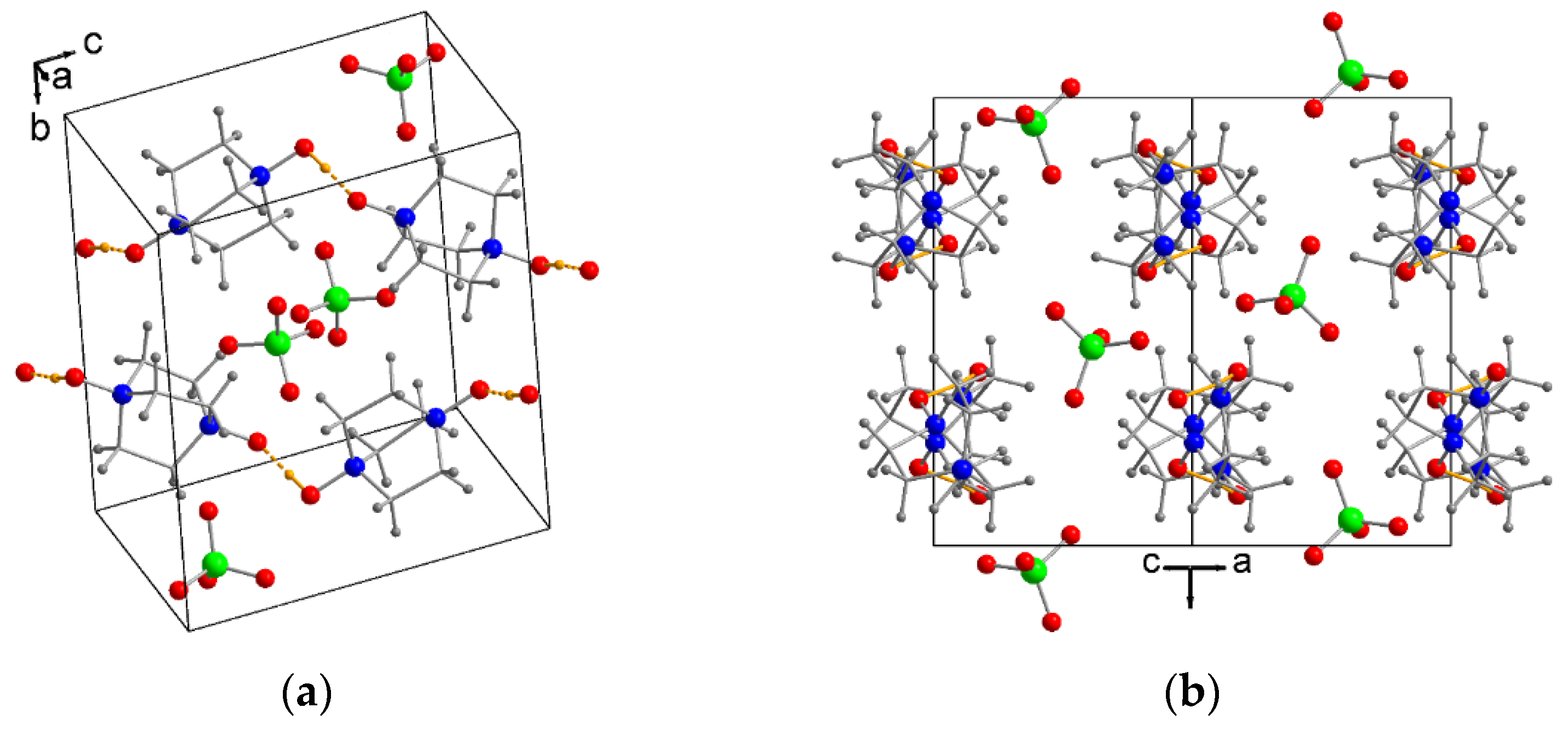
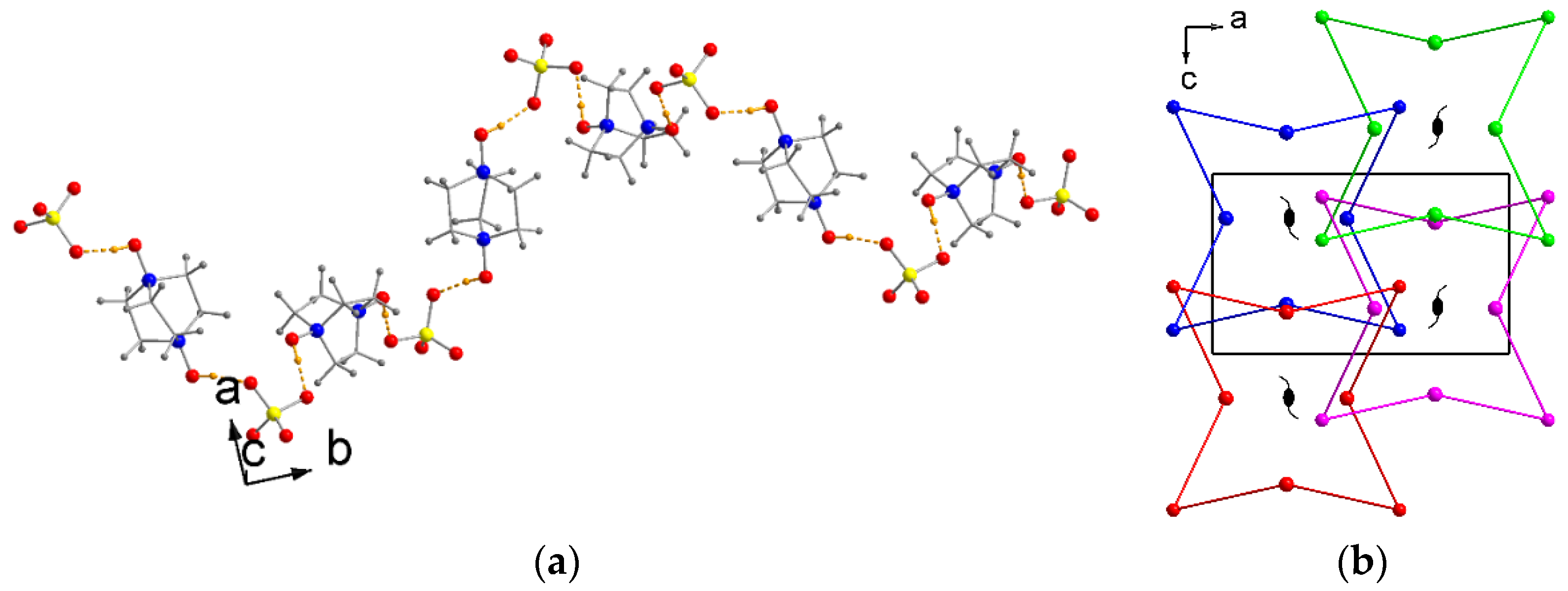
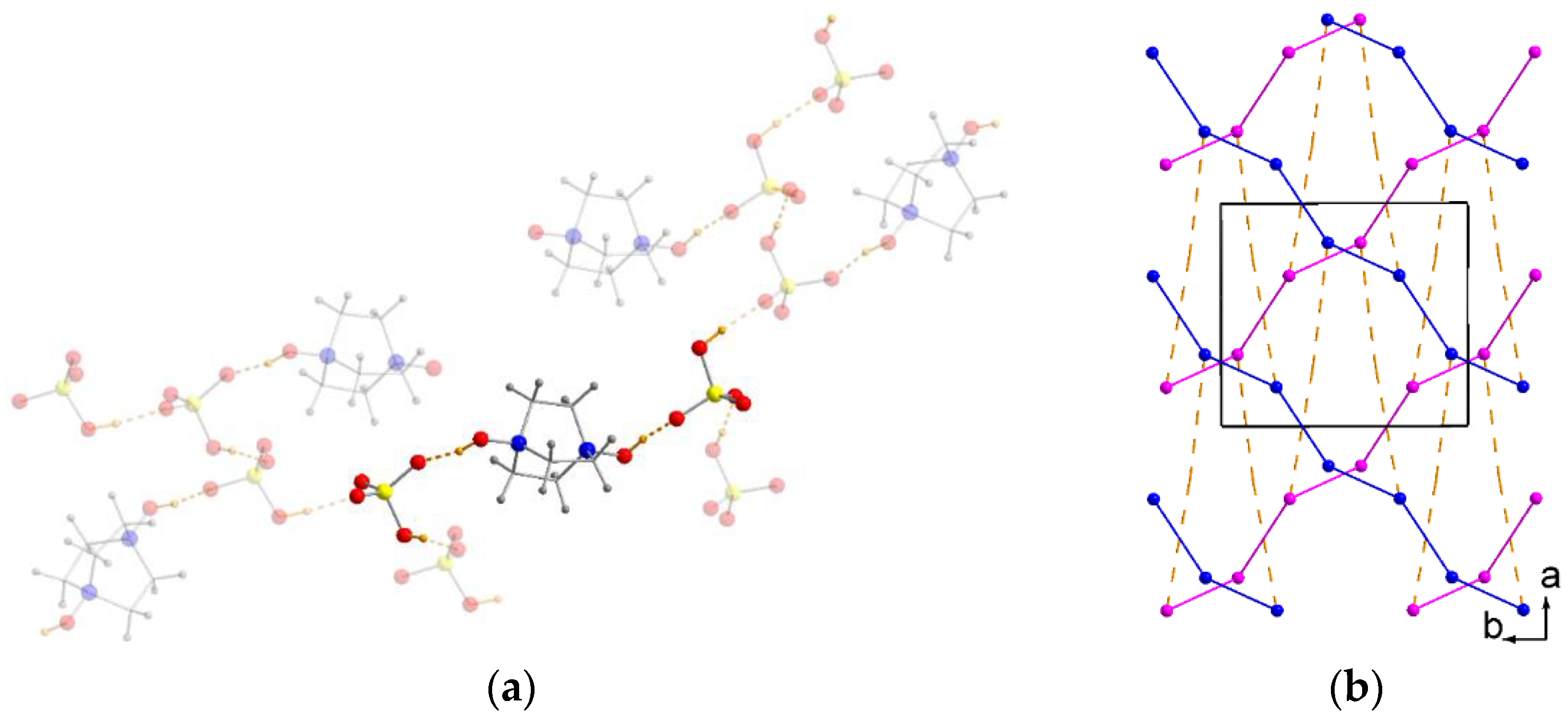
2.1. Crystal Structures
2.2. Characterization
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of Odabco·2HNO3 (1)
3.4. Synthesis of Odabco·HClO4 (2)
3.5. Synthesis of Odabco·H2SO4 (3)
3.6. Synthesis of Odabco·2H2SO4 (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Crystallographic Data
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Chemical formula | C6H14N4O8 | C6H13ClN2O6 | C6H14N2O6S | C6H16N2O10S2 |
| Mr, g/mol | 270.21 | 244.63 | 242.25 | 340.33 |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic | Monoclinic |
| Space group | P21/n | P21/n | Pnna | Cc |
| Temperature, K | 150 | 150 | 135 | 150 |
| a, Å | 6.6537(4) | 7.8923(4) | 11.6565(7) | 9.5987(3) |
| b, Å | 18.9836(11) | 11.6883(4) | 23.8750(14) | 10.3468(3) |
| c, Å | 8.5758(5) | 10.6752(4) | 7.0514(4) | 12.7952(5) |
| α, ° | 90 | 90 | 90 | 90 |
| b, ° | 94.448(6) | 98.338(4) | 90 | 102.635(4) |
| γ, ° | 90 | 90 | 90 | 90 |
| V, Å3 | 1079.96(11) | 974.35(7) | 1962.4(2) | 1239.99(7) |
| Z | 4 | 4 | 8 | 4 |
| F(000) | 568 | 512 | 1024 | 712 |
| D(calc.), g·cm–3 | 1.662 | 1.668 | 1.640 | 1.823 |
| μ, mm–1 | 0.15 | 0.41 | 0.34 | 0.49 |
| Crystal size, mm | 0.53 × 0.30 × 0.29 | 0.70 × 0.60 × 0.34 | 0.68 × 0.26 × 0.15 | 0.53 × 0.35 × 0.26 |
| θ range for data collection, ° | 2.2 < θ < 25.3 | 2.6 < θ < 25.4 | 3.4 < θ < 25.4 | 2.9 < θ < 25.4 |
| No. of reflections: measured/independent/observed [I > 2σ(I)] | 8569/ 1974/ 1730 | 6851/ 1783/ 1622 | 5377/ 1796/ 1564 | 9464/ 2296/ 2258 |
| Rint | 0.0213 | 0.0222 | 0.0168 | 0.0201 |
| Index ranges | –7 ≤ h ≤ 8 –22 ≤ k ≤ 22 –10 ≤ l ≤ 18 | –9 ≤ h ≤ 6 –14 ≤ k ≤ 14 –12 ≤ l ≤ 12 | –12 ≤ h ≤ 14 –20 ≤ k ≤ 28 –8 ≤ l ≤ 6 | –11 ≤ h ≤ 11 –12 ≤ k ≤ 12 –15 ≤ l < 15 |
| Final R indices [I > 2σ(I)] | R1 = 0.0327 wR2 = 0.0818 | R1 = 0.0308 wR2 = 0.0845 | R1 = 0.0314 wR2 = 0.0792 | R1 = 0.0218 wR2 = 0.0558 |
| Final R indices (all data) | R1 = 0.0388 wR2 = 0.0853 | R1 = 0.0343 wR2 = 0.0866 | R1 = 0.0381 wR2 = 0.0824 | R1 = 0.0222 wR2 = 0.0562 |
| Goodness of fit on F2 | 1.073 | 1.068 | 1.060 | 1.098 |
| Largest diff. peak, hole, e/Å3 | 0.22, –0.26 | 0.26, –0.39 | 0.28, –0.41 | 0.16, –0.31 |
References
- Hon, P.K.; Mak, T.C.W. Isolation and crystal structures of 1:3 molecular complexes of triethylenediamineN,N′-dioxide with hydrogen peroxide and water. J. Crystallogr. Spectrosc. Res. 1987, 17, 419–429. [Google Scholar] [CrossRef]
- Harmon, K.M.; Akin, A.C. Hydrogen bonding Part 40. IR and thermodynamic study of stability and stoichiometry for triethylenediamine dioxide hydrates, triethylenediamine monohydrate, and a mixed H2O/H2O2 solvate of triethylenediamine dioxide. J. Mol. Struct. 1992, 265, 59–73. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Churakov, A.V.; Prikhodchenko, P.V.; Lev, O.; Vener, M.V. Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions. Molecules 2021, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Saraswatula, V.G.; Bhat, M.A.; Gurunathana, P.K.; Saha, B.K. Comparison of pyridyl and pyridyl N-oxide groups as acceptor in hydrogen bonding with carboxylic acid. CrystEngComm 2014, 16, 4715–4721. [Google Scholar] [CrossRef]
- Sun, F.-X.; Zhu, G.-S.; Fang, Q.-R.; Qiu, S.-L. A novel 3D metal-organic framework with the pcu topology constructed from 1,4-diaza-bicyclo[2.2.2]octane-N,N′-dioxide. Inorg. Chem. Comm. 2007, 10, 649. [Google Scholar] [CrossRef]
- Abasheeva, K.D.; Demakov, P.A.; Dybtsev, D.N.; Fedin, V.P. Crystal Structure of Coordination cobalt(II) and zinc(II) Polymers with 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide. J. Struct. Chem. 2022, 63, 1349–1357. [Google Scholar] [CrossRef]
- Zheng, B.; Luo, J.; Wang, F.; Peng, Y.; Li, G.; Huo, Q.; Liu, Y. Construction of Six Coordination Polymers Based on a 5,5′-(1,2-Ethynyl)bis-1,3-benzenedicarboxylic Ligand: Synthesis, Structure, Gas Sorption, and Magnetic Properties. Cryst. Growth Des. 2013, 13, 1033–1044. [Google Scholar] [CrossRef]
- Demakov, P.A.; Romanov, A.S.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Synthesis and structure of manganese(II) coordination polymers with 1,4-diazabicyclo[2.2.2]octane N, N′-dioxide: Solvent and template effects. Russ. Chem. Bull. 2020, 69, 1511–1519. [Google Scholar] [CrossRef]
- Liao, W.-Q.; Zhou, Q.-Q.; Zhang, Y. catena-Poly[1,4-dihy droxy-1,4-di azoniabicyclo [2.2.2]octane[aqua tri-[mu]-chlorido-trichloridodicuprate(II)]]. Acta Crystallogr. 2013, C69, 380–383. [Google Scholar] [CrossRef]
- Chen, L.Z.; Sun, J. Reversible ferroelectric phase transition of 1,4-diazabicyclo[2,2,2]octane N,N′-dioxide di(perchlorate). Inorg. Chem. Comm. 2017, 76, 67–70. [Google Scholar] [CrossRef]
- Leblanc, N.; Allain, M.; Mercier, N.; Cariati, E. Protonated N,N′-Dioxide-4,4′-bipyridine, an Interesting Synthon for the Building of Polar H-Bonded Networks? Cryst. Growth Des. 2011, 11, 5200–5205. [Google Scholar] [CrossRef][Green Version]
- Ye, H.-Y.; Zhang, Y.; Noro, S.-I.; Kubo, K.; Yoshitake, M.; Liu, Z.-Q.; Cai, H.-L.; Fu, D.-W.; Yoshikawa, H.; Awaga, K.; et al. Molecule-displacive ferroelectricity in organic supramolecular solids. Sci. Rep. 2013, 3, 2249. [Google Scholar] [CrossRef] [PubMed]
- Sandipan Roy, S.; Biradha, K. Exploration of Salts and Cocrystals of 2,2′,6,6′-Tetracarboxybiphenyl with Acetic Acid, Monobasic and Dibasic N-Heterocycles, and N-Oxides. Cryst. Growth Des. 2013, 13, 3232–3241. [Google Scholar] [CrossRef]
- Chen, L.; Ji, Q.; Wang, X.; Pan, Q.; Cao, X.; Xu, G. Two novel metal–organic coordination polymers based on ligand 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide with phase transition, and ferroelectric and dielectric properties. CrystEngComm 2017, 19, 5907–5914. [Google Scholar] [CrossRef]
- Demakov, P.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Zinc(II) metal-organic frameworks with 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide: Control of the parameters of the cationic porous framework and optical properties. Russ. Chem. Bull. 2022, 71, 83–90. [Google Scholar] [CrossRef]
- Demakov, P.A.; Yudina, Y.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Crystal structure of zinc coordination polymers based on 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide: Effect of hydrophobicity of carboxylate ligands. J. Struct. Chem. 2021, 62, 403–411. [Google Scholar] [CrossRef]
- Periasamy, A.; Muruganand, S.; Palaniswamy, M. Vibrational Studies of Na2SO4, K2SO4, NaHSO4 AND KHSO4 crystals. Rasayan J. Chem. 2009, 2, 981–989. [Google Scholar]
- CrysAlisPro 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015; Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 2 November 2022).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abasheeva, K.D.; Demakov, P.A.; Fedin, V.P. Diverse Hydrogen-Bonded Structural Motifs in 1,4-Diazabicyclo[2.2.2]octane N,N’-Dioxide Salts with Oxoanions. Molbank 2022, 2022, M1508. https://doi.org/10.3390/M1508
Abasheeva KD, Demakov PA, Fedin VP. Diverse Hydrogen-Bonded Structural Motifs in 1,4-Diazabicyclo[2.2.2]octane N,N’-Dioxide Salts with Oxoanions. Molbank. 2022; 2022(4):M1508. https://doi.org/10.3390/M1508
Chicago/Turabian StyleAbasheeva, Ksenia D., Pavel A. Demakov, and Vladimir P. Fedin. 2022. "Diverse Hydrogen-Bonded Structural Motifs in 1,4-Diazabicyclo[2.2.2]octane N,N’-Dioxide Salts with Oxoanions" Molbank 2022, no. 4: M1508. https://doi.org/10.3390/M1508
APA StyleAbasheeva, K. D., Demakov, P. A., & Fedin, V. P. (2022). Diverse Hydrogen-Bonded Structural Motifs in 1,4-Diazabicyclo[2.2.2]octane N,N’-Dioxide Salts with Oxoanions. Molbank, 2022(4), M1508. https://doi.org/10.3390/M1508








