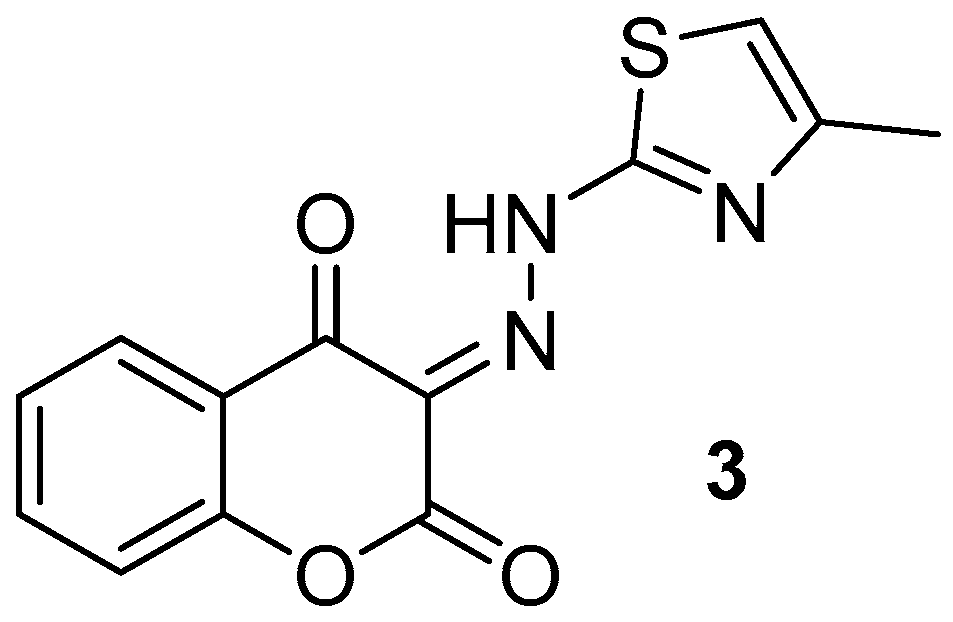
(E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione
Abstract
1. Introduction
2. Results and Discussion
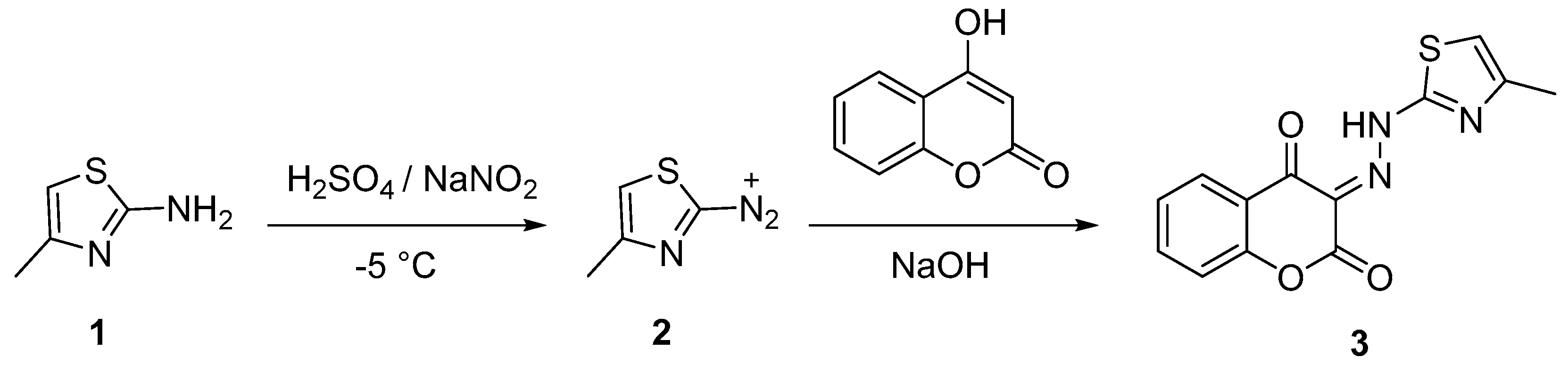
2.1. Synthesis of 3
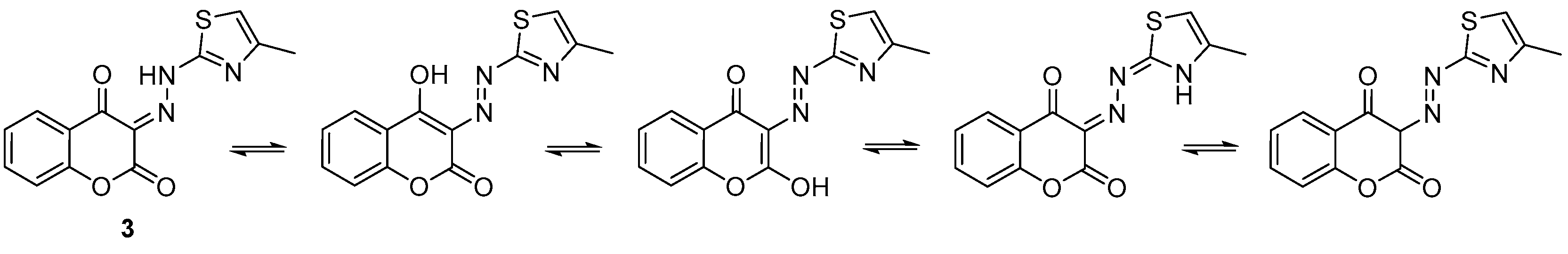
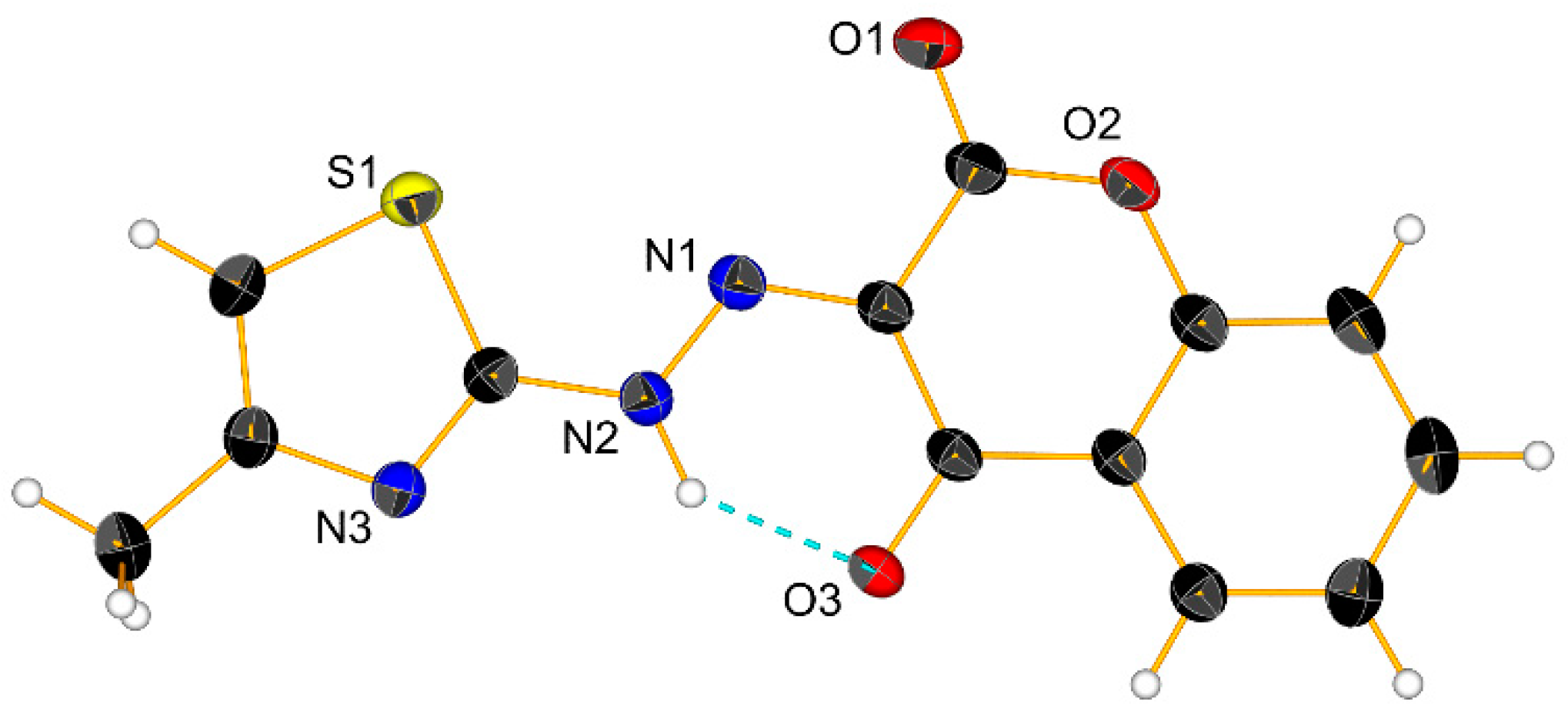
2.2. X-ray Crystal Structure
3. Materials and Methods
3.1. General Comments
3.2. Synthesis of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akchurin, I.O.; Yakhutina, A.I.; Bochkov, A.Y.; Solovjova, N.P.; Medvedev, M.G.; Traven, V.F. Novel push-pull fluorescent dyes–7-(diethylamino)furo- and thieno[3,2-c]coumarins derivatives: Structure, electronic spectra and TD-DFT study. J. Mol. Struct. 2018, 1160, 215–221. [Google Scholar] [CrossRef]
- Erdogdu, Y.; Baskose, U.C.; Saglam, S.; Erdogdu, M.; Ogutcu, H.; Özçelik, S. Structural, thermal, spectroscopic, electronic and biological activity properties of coumarin-153 dyes for DSSCs: A DFT benchmark study. J. Mol. Struct. 2020, 1221, 128873. [Google Scholar] [CrossRef]
- Nagaraja, O.; Bodke, Y.D.; Kenchappa, R.; Ravi Kumar, S. Synthesis and characterization of 3-[3-(1H-benzimidazol-2-ylsulfanyl)-3-phenyl propanoyl]-2H-chromen-2-one derivatives as potential biological agents. Chem. Data Collect 2020, 27, 100369. [Google Scholar] [CrossRef]
- Nagaraja, O.; Bodke, Y.D.; Pushpavathi, I.; Ravi Kumar, S. Synthesis, characterization and biological investigations of potentially bioactive heterocyclic compounds containing 4-hydroxy coumarin. Heliyon 2020, 6, e04245. [Google Scholar] [CrossRef] [PubMed]
- Panitsiri, A.; Tongkhan, S.; Radchatawedchakoon, W.; Sakee, U. Synthesis and anion recognition studies of novel bis (4-hydroxycoumarin) methane azo dyes. J. Mol. Struct. 2016, 1107, 14–18. [Google Scholar] [CrossRef]
- Jashari, A.; Imeri, F.; Ballazhi, L.; Shabani, A.; Mikhova, B.; Dräger, G.; Popovski, E.; Huwiler, A. Synthesis and cellular characterization of novel isoxazolo- and thiazolohydrazinylidene-chroman-2,4-diones on cancer and non-cancer cell growth and death. Bioorg. Med. Chem. 2014, 22, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef] [PubMed]
- Stamboliyska, B.; Jashari, A.; Yancheva, D.; Mikhova, B.; Batovska, D.; Popovski, E.; Mladenovska, K. Structure and radical scavenging activity of isoxazolo- and thiazolohydrazinylidenechroman-2,4-diones. Bulg. Chem. Commun. 2017, 49, 99–105. [Google Scholar]
- Jashari, A.; Popovski, E.; Mikhova, B.; Nikolova, R.P.; Shivachev, B.L. 3-[2-(5-tert-Butyl-1,2-oxazol-3-yl)hydrazinyl-idene]chroman-2,4-dione. Acta Crystallogr. E 2013, 69, o258. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Bodke, Y.D.; Nagaraja, O.; Lohith, T.N.; Nagaraju, G.; Sridhar, M.A. Coumarin-Benzothiazole Based Azo Dyes: Synthesis, Characterization, Computational, Photophysical and Biological Studies. J. Mol. Struct. 2021, 1246, 131170. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, F.; Crochet, A.; Zobi, F. (E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione. Molbank 2022, 2022, M1504. https://doi.org/10.3390/M1504
Rahmani F, Crochet A, Zobi F. (E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione. Molbank. 2022; 2022(4):M1504. https://doi.org/10.3390/M1504
Chicago/Turabian StyleRahmani, Fatlinda, Aurélien Crochet, and Fabio Zobi. 2022. "(E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione" Molbank 2022, no. 4: M1504. https://doi.org/10.3390/M1504
APA StyleRahmani, F., Crochet, A., & Zobi, F. (2022). (E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione. Molbank, 2022(4), M1504. https://doi.org/10.3390/M1504






