Synthesis and Crystal Structures of Halogen-Substituted 2-Aryl-N-phenylbenzimidazoles
Abstract
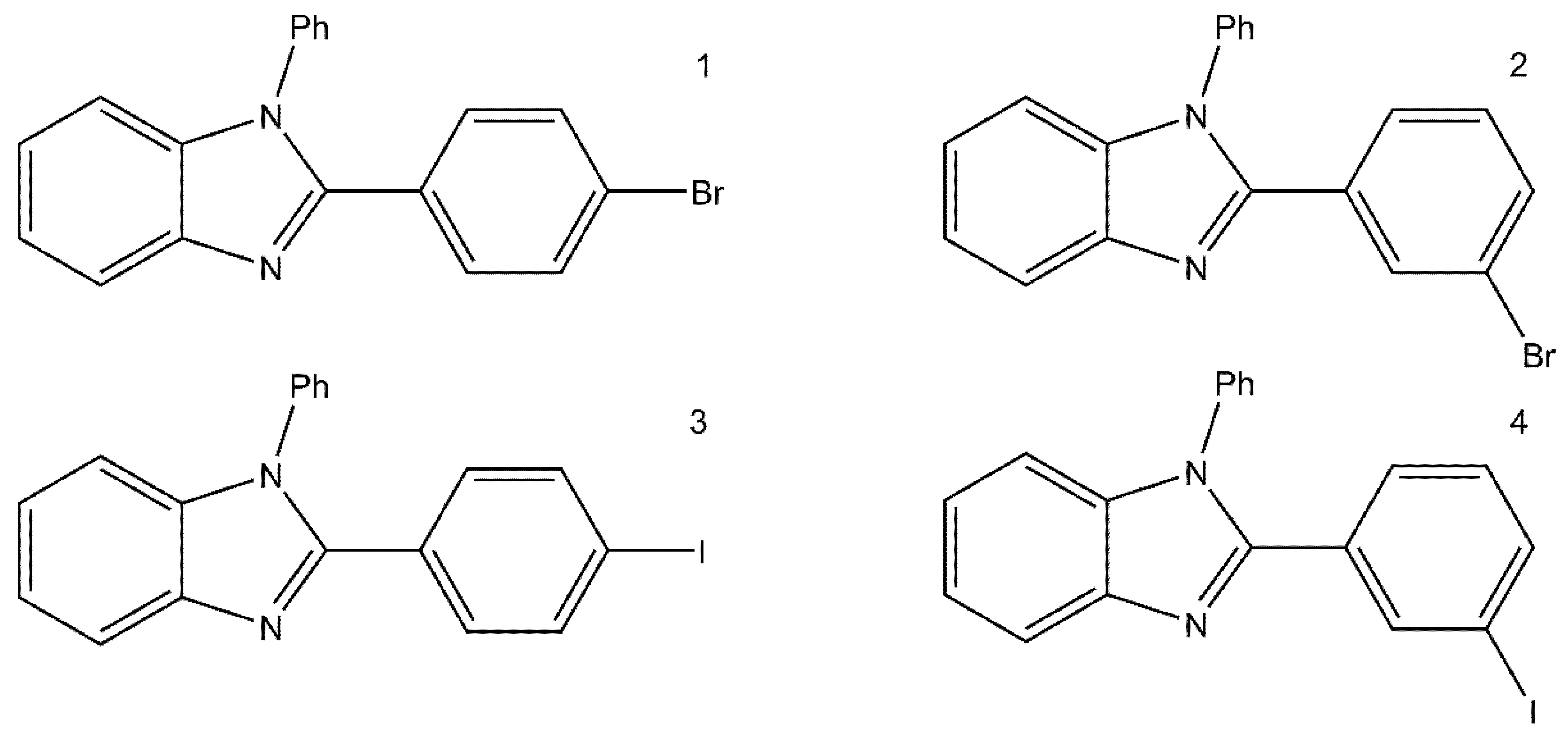
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Comment
3.2. Synthesis
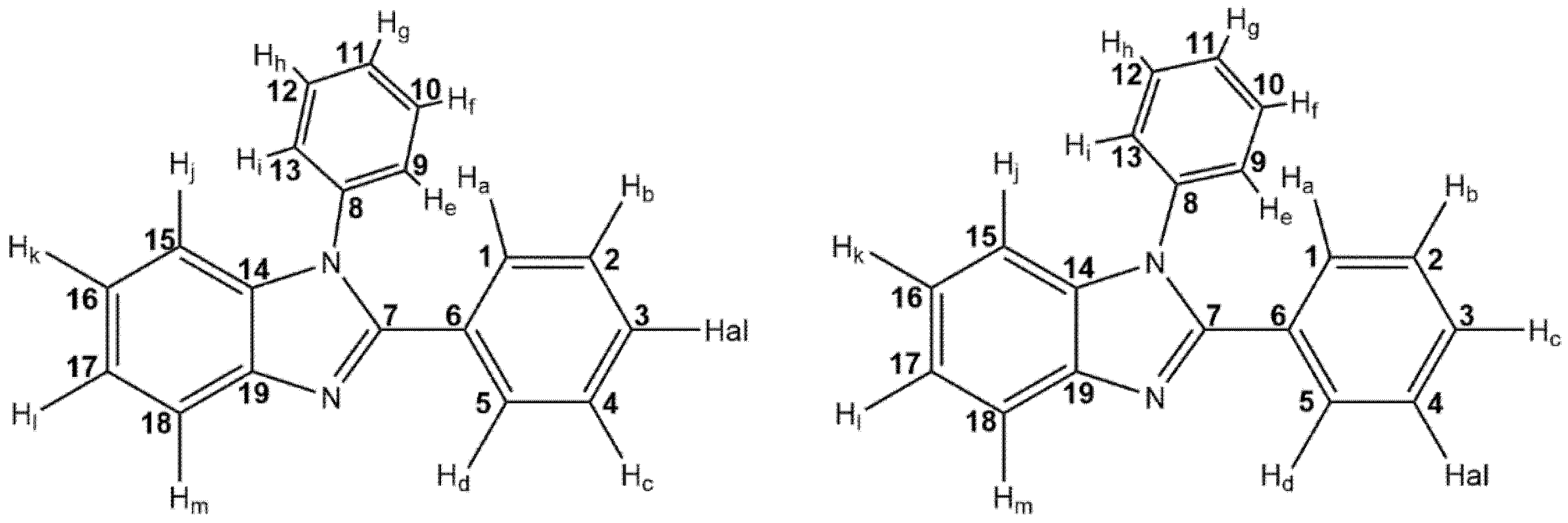
3.3. Crystallography Details
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasava, M.S.; Bhoi, M.N.; Rathwa, S.K.; Jethava, D.J.; Acharya, P.T.; Patel, D.B.; Patel, H.D. Benzimidazole: A milestone in the field of medicinal chemistry. Mini-Rev. Med. Chem. 2020, 20, 532–565. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, H.; Tejada-Rodríguez, C.J.; Leyva-Ramos, S. A Panoramic Review of Benzimidazole Derivatives and Their Potential Biological Activity. Mini-Rev. Med. Chem. 2022, 22, 1268–1280. [Google Scholar] [CrossRef]
- Pathare, B.; Bansode, T. Review—Biological Active Benzimidazole Derivatives. Results Chem. 2021, 3, 100200. [Google Scholar] [CrossRef]
- Leila, D.; Gokhan, Z.; Mir, B.B. Cholinesterases inhibitory activity of 1H-benzimidazole derivatives. Biointerface Res. Appl. Chem. 2020, 11, 10739–10745. [Google Scholar] [CrossRef]
- Panda, S.; Malik, R.; Jain, S.C. Synthetic approaches to 2-arylbenzimidazoles: A review. Curr. Org. Chem. 2012, 16, 1905–1919. [Google Scholar] [CrossRef]
- Basha, N.J. Therapeutic efficacy of benzimidazole and its analogs: An update. Polycycl. Aromat. Compd. 2022, 1–21. [Google Scholar] [CrossRef]
- G, A.C.; Gondru, R.; Li, Y.; Banothu, J. Coumarin–benzimidazole hybrids: A review of developments in medicinal chemistry. Eur. J. Med. Chem. 2022, 227, 113921. [Google Scholar] [CrossRef]
- Shatokhin, S.S.; Tuskaev, V.A.; Gagieva, S.C.; Markova, A.A.; Pozdnyakov, D.I.; Melnikova, E.K.; Bulychev, B.M.; Oganesyan, E.T. Synthesis, Cytotoxic and antioxidant activities of new n-substituted 3-(benzimidazol-2-Yl)-chromones containing 2,6-di-Tert-butylphenol fragment. J. Mol. Struct. 2022, 1249, 131683. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Zhukova, N.A. Recent developments towards synthesis of (Het) arylbenzimidazoles. Synthesis 2021, 53, 1849–1878. [Google Scholar] [CrossRef]
- Sigh, K.S.; Joy, F.; Devi, P. Ruthenium(II)-catalyzed synthesis of 2-arylbenzimidazole and 2-arylbenzothiazole in water. Transit. Met. Chem. 2021, 46, 181–190. [Google Scholar] [CrossRef]
- Tzani, M.A.; Gabriel, C.; Lykakis, I.N. Selective synthesis of benzimidazoles from o-phenylenediamine and aldehydes promoted by supported gold nanoparticles. Nanomaterials 2020, 10, 2405. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, Z.A.; Acharya, P.T.; Jethava, D.J.; Patel, D.B.; Vasava, M.S.; Rajani, D.P.; Pithawala, E.; Patel, H.D. Microwave assisted synthesis, biological activities, and in silico investigation of some benzimidazole derivatives. J. Heterocycl. Chem. 2020, 57, 4215–4238. [Google Scholar] [CrossRef]
- Ridley, H.F.; Spickett, R.G.W.; Timmis, G.M. A new synthesis of benzimidazoles and aza-analogs. J. Heterocycl. Chem. 1965, 2, 453–456. [Google Scholar] [CrossRef]
- Lavrova, M.A.; Mishurinskiy, S.A.; Smirnov, D.E.; Kalle, P.; Krivogina, E.V.; Kozyukhin, S.A.; Emets, V.V.; Mariasina, S.S.; Dolzhenko, V.D.; Bezzubov, S.I. Cyclometalated Ru (Ii) Complexes with tunable redox and optical properties for dye-sensitized solar cells. Dalt. Trans. 2020, 49, 16935–16945. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Guedes, R.C.; Pereira, M.M. Synthesis of computationally designed 2,5(6) -benzimidazole derivatives via pd-catalyzed reactions for potential, e. coli dna gyrase b inhibition. Molecules 2021, 26, 1326. [Google Scholar] [CrossRef]
- Yellol, J.; Pérez, S.A.; Buceta, A.; Yellol, G.; Donaire, A.; Szumlas, P.; Bednarski, P.J.; Makhloufi, G.; Janiak, C.; Espinosa, A.; et al. Novel c,n-cyclometalated benzimidazole ruthenium(ii) and iridium(iii) complexes as antitumor and antiangiogenic agents: A structure–activity relationship study. J. Med. Chem. 2015, 58, 7310–7327. [Google Scholar] [CrossRef]
- Munnik, B.L.; Kaschula, C.H.; Watson, D.J.; Wiesner, L.; Loots, L.; Chellan, P. Synthesis and study of organometallic pgm complexes containing 2-(2-pyridyl) benzimidazole as antiplasmodial agents. Inorg. Chim. Acta 2022, 540, 121039. [Google Scholar] [CrossRef]
- Laha, P.; Husain, A.; Patra, S. Tuning the emission maxima of iridium systems using benzimidazole-based cyclometallating Framework. J. Mol. Liq. 2022, 349, 118446. [Google Scholar] [CrossRef]
- Buil, M.L.; Esteruelas, M.A.; López, A.M. Recent advances in synthesis of molecular heteroleptic osmium and iridium phosphorescent emitters. Eur. J. Inorg. Chem. 2021, 2021, 4731–4761. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Kiselev, Y.M.; Churakov, A.V.; Kozyukhin, S.A.; Sadovnikov, A.A.; Grinberg, V.A.; Emets, V.V.; Doljenko, V.D. Iridium (III) 2-phenylbenzimidazole complexes: Synthesis, structure, optical properties, and applications in dye-sensitized solar cells. Eur. J. Inorg. Chem. 2016, 2016, 347–354. [Google Scholar] [CrossRef]
- Wang, L.; Cui, P.; Lystrom, L.; Lu, J.; Kilina, S.; Sun, W. Heteroleptic cationic iridium (iii) complexes bearing phenanthroline derivatives with extended π-conjugation as potential broadband reverse saturable absorbers. New J. Chem. 2019, 44, 456–465. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Zharinova, I.S.; Khusyainova, A.A.; Kiselev, Y.M.; Taydakov, I.V.; Varaksina, E.A.; Metlin, M.T.; Tobohova, A.S.; Korshunov, V.M.; Kozyukhin, S.A.; et al. Aromatic beta-diketone as a novel anchoring ligand in iridium (iii) complexes for dye-sensitized solar cells. Eur. J. Inorg. Chem. 2020, 2020, 3277–3286. [Google Scholar] [CrossRef]
- Largeron, M.; Nguyen, K. Recent advances in the synthesis of benzimidazole derivatives from the oxidative coupling of primary amines. Synthesis 2018, 50, 241–253. [Google Scholar] [CrossRef]
- Brunen, S.; Grell, Y.; Steinlandt, P.S.; Harms, K.; Meggers, E. Bis-cyclometalated indazole and benzimidazole chiral-at-iridium complexes: Synthesis and asymmetric catalysis. Molecules 2021, 26, 1822. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Siamaki, A.R.; Belecki, K.; Gupton, B.F. A convergent approach to the total synthesis of telmisartan via a suzuki cross-coupling reaction between two functionalized benzimidazoles. J. Org. Chem. 2015, 80, 1915–1919. [Google Scholar] [CrossRef]
- Bezzubov, S.I.; Doljenko, V.D.; Troyanov, S.I.; Kiselev, Y.M. Tuning the photophysical and electrochemical properties of iridium(iii)2-aryl-1-phenylbenzimidazole complexes. Inorg. Chim. Acta 2014, 415, 22–30. [Google Scholar] [CrossRef]
- Smirnov, D.E.; Tatarin, S.V.; Bezzubov, S.I. Synthesis and crystal structures of n -h, n -phenyl and n -benzyl-2-(4-hexyloxyphenyl)benzimidazoles. Acta Crystallogr. Sect. E Crystallogr. Commun. 2021, 77, 618–622. [Google Scholar] [CrossRef]
- Kassim, K.; Hashim, N.Z.N.; Fadzil, A.H.; Yusof, M.S.M. 2-(4-Chlorophenyl)-1-phenyl-1 H -benzimidazole. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o799. [Google Scholar] [CrossRef]
- Su, Y.-J.; Huang, H.-L.; Li, C.-L.; Chien, C.-H.; Tao, Y.-T.; Chou, P.-T.; Datta, S.; Liu, R.-S. Highly efficient red electrophosphorescent devices based on iridium isoquinoline complexes: Remarkable external quantum efficiency over a wide range of current. Adv. Mater. 2003, 15, 884–888. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A Found. Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koptyaeva, A.G.; Zakharov, A.Y.; Kiseleva, M.A.; Mariasina, S.S.; Kalle, P.; Churakov, A.V.; Bezzubov, S.I. Synthesis and Crystal Structures of Halogen-Substituted 2-Aryl-N-phenylbenzimidazoles. Molbank 2022, 2022, M1498. https://doi.org/10.3390/M1498
Koptyaeva AG, Zakharov AY, Kiseleva MA, Mariasina SS, Kalle P, Churakov AV, Bezzubov SI. Synthesis and Crystal Structures of Halogen-Substituted 2-Aryl-N-phenylbenzimidazoles. Molbank. 2022; 2022(4):M1498. https://doi.org/10.3390/M1498
Chicago/Turabian StyleKoptyaeva, Anastasia G., Alexander Y. Zakharov, Marina A. Kiseleva, Sofia S. Mariasina, Paulina Kalle, Andrei V. Churakov, and Stanislav I. Bezzubov. 2022. "Synthesis and Crystal Structures of Halogen-Substituted 2-Aryl-N-phenylbenzimidazoles" Molbank 2022, no. 4: M1498. https://doi.org/10.3390/M1498
APA StyleKoptyaeva, A. G., Zakharov, A. Y., Kiseleva, M. A., Mariasina, S. S., Kalle, P., Churakov, A. V., & Bezzubov, S. I. (2022). Synthesis and Crystal Structures of Halogen-Substituted 2-Aryl-N-phenylbenzimidazoles. Molbank, 2022(4), M1498. https://doi.org/10.3390/M1498






