Abstract
A chromeno[4,3-d]pyrazolo[3,4-b]pyridinone derivative 3 bearing thieno[2,3-d]pyrimidine moiety, 5-methyl-1-phenyl-3-(thieno[2,3-d]pyrimidin-4-yl)chromeno[4,3-d]pyrazolo[3,4-b]pyridin-6(3H)-one, was efficiently prepared in 75% yield by the reaction of 3-phenyl-1-(thieno[2,3-d]pyrimidin-4-yl)-1H-pyrazol-5-amine 1 with 3-acetyl-2H-chromen-2-one 2 in the presence of FeCl3-SiO2 as a catalyst in refluxing ethanol for 6 h. The structure of the new synthesized compound was characterized by 1H-, 13C- NMR, IR spectroscopy, mass-spectrometry, and elemental analysis.
1. Introduction
Many coumarin-based derivatives have been synthesized and explored due to considerable attention to their biological properties and therapeutic potentials. They displayed diverse biological and pharmacological activities, such as antimicrobial [1], anticonvulsant [2], anticancer [3], and antiviral [4] activities. Coumarin derivatives with intramolecular charge transfer character have also been investigated and applied in fluorescence sensors [5,6]. Pyrazolo[3,4-b]pyridine and its derivatives are known to have a wide range of biological activities such as anticancer [7], anti-inflammatory [8], and neuroactive agent [9]. Fused pyrazolo[3,4-b]pyridines showed photochemical properties of high fluorescence [10]. On the other hand, thieno[2,3-d]pyrimidine derivatives [11] continue to attract considerable interest in medicinal chemistry because of remarkable biological properties such as antibacterial [12], anticancer [13], antimalarial [14], and anti-inflammatory [15,16] activities. However, there are only a few reports on the synthesis of fused coumarin-pyrazolo[3,4-b]pyridine derivatives [17,18]. We report herein the synthesis of a hybrid compound 3 associated with these two pharmacophores, bearing thieno[2,3-d]pyrimidine moiety.
2. Results
The target compound 3 was prepared as shown in Scheme 1. The starting material 1, 3-phenyl-1-(thieno[2,3-d]pyrimidin-4-yl)-1H-pyrazol-5-amine, was obtained according to the previously reported procedure [19]. When 1 was allowed to react with 3-acetyl-2H-chromen-2-one 2 in refluxing ethanol for 6 h in the presence of FeCl3 adsorbed on silica gel [20] as a catalyst, a fused tetracyclic compound 3, 5-methyl-1-phenyl-3-(thieno[2,3-d]pyrimidin-4-yl)chromeno[4,3-d]pyrazolo[3,4-b]pyridin-6(3H)-one, was formed in 75% yield. The use of the heterogeneous catalyst, FeCl3-SiO2, for this reaction has the advantages of low cost, easy work-up, and enhanced yield compared to FeCl3 alone (69% yield) or other catalysts [18]. The 1H-NMR spectrum of 3 showed exhibited the expected pattern with a sharp singlet δ 9.24 ppm attributed to a pyrimidine proton and two doublets δ 7.94 and 7.63 (J = 5.7 Hz, respectively) ppm for thiophene protons in thieno[2,3-d]pyrimidine ring (Supplementary Materials). Two multiplet signals from the protons of phenyl bonded to the pyrazole ring were observed at δ 7.60—7.58 and 7.48—7.44 ppm, respectively. It is noteworthy that one of the aromatic protons of the coumarin ring appeared at δ 6.73 (t, 1H, J = 7.4 Hz) in a higher field, whereas others were found at δ 7.52 (m, 1H), 7.38 (d, 1H, J = 7.3 Hz) and 7.34 (d, 1H, J = 7.4 Hz), respectively. This may be attributed to the through-space anisotropic effect of the phenyl group attached to the pyrazole ring on this proton of the coumarin ring. It presented a sharp signal at δ 3.25 ppm attributed to methyl proton in the pyridine ring of 3. In the 13C-NMR spectrum, compound 3 showed a peak δ 28.5 ppm for a methyl carbon, including six carbons for thienopyrimidine ring and tetracyclic carbons having a phenyl group shown at δ 171.8–108.1 ppm. The IR spectrum revealed a characteristic CO absorption at 1722 cm−1. The mass spectrum showed m/z = 461 (M+) corresponding to the molecular formula, C26H15N5O2S. The elemental analysis also provided satisfactory results.
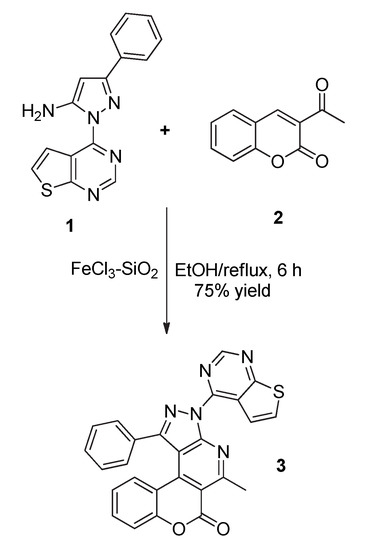
Scheme 1.
Synthesis of the target compound 3.
In conclusion, a hybrid compound 3, fused coumarin-pyrazolo[3,4-b]pyridine derivative bearing thieno[2,3-d]pyrimidine moiety, was synthesized efficiently by the reaction of 3-phenyl-1-(thieno[2,3-d]pyrimidin-4-yl)-1H-pyrazol-5-amine 1 with 3-acetyl-2H-chromen-2-one 2 in the presence of FeCl3-SiO2 in refluxing ethanol. This compound could be useful as a potential material having biological activities or photochemical properties of fluorescence.
3. Materials and Methods
3.1. General Information
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and TCI (Tokyo, Japan). The solvents used were purified using standard techniques. Melting point was determined on Kofler apparatus. Thin-layer chromatography (TLC) was used to monitor reactions and performed using aluminum sheets precoated with silica gel 60 (HF254, Merck, Waltham, MA, USA) and visualized with UV radiation (Fisher Scientific, Waltham, MA, USA). The 1H- and 13C- NMR spectra were recorded in deuterated chloroform with TMS as the standard on a Bruker Avancell 500 FT-NMR. The IR spectrum was recorded on Bruker Invenio FT-IR (Billerica, MA, USA). The mass spectrum was obtained with Agilent 6890 Mstation instrument (Santa Clara, CA, USA).
3.2. Synthesis of 5-Methyl-1-phenyl-3-(thieno[2,3-d]pyrimidin-4-yl)chromeno[4,3-d]pyrazolo-[3,4-b]pyridin-6(3H)-one (3)
A solution of 3-phenyl-1-(thieno[2,3-d]pyrimidin-4-yl)-1H-pyrazol-5-amine 1 (293 mg, 1.0 mmol), 3-acetyl-2H-chromen-2-one 2 (188 mg, 1.0 mmol), and FeCl3-SiO2 (176 mg, 0.01 mmol FeCl3) in ethanol (20 mL) was refluxed at 80 °C with stirring. After completion of the reaction (6 h, monitored by TLC), the mixture was cooled, filtered, and washed with ethanol. The filtrate was evaporated in vacuo, and the collected solid was recrystallized from chloroform to give light yellow solid of 3 in 75% yield (345 mg). Mp 294–295 °C; TLC Rf = 0.42 (fluorescent blue spot, dichloromethane:MeOH = 40:1). 1H-NMR (500 MHz, CDCl3) (ppm) δ 9.24 (s, 1H), 7.94 (d, J = 5.7 Hz, 1H), 7.63 (d, J = 5.7 Hz, 1H), 7.60–7.58 (m, 2H), 7.52 (m, 1H), 7.48–7.44 (m, 3H), 7.38 (d, 1H, J = 7.3 Hz), 7.34 (d, 1H, J = 7.4 Hz), 6.73 (t, 1H, J = 7.4 Hz), 3.25 (s, H). 13C-NMR (125 MHz, CDCl3) (ppm) δ 171.8, 165.5, 159.6, 153.5, 153.1, 152.8, 151.6, 149.9, 142.5, 133.6, 133.1, 131.6, 129.8, 129.6, 127.4, 123.1, 123.0, 122.2, 117.0, 115.4, 112.6, 108.1, 28.5. IR (KBr) 1722, 1429 cm−1. MS (EI) m/z = 461 (M+, 100%). Anal. calc. for C26H15N5O2S, %: C, 67.67; H, 3.28; N, 15.18. Found, %: C, 67.78; H, 3.11; N, 15.29.
Supplementary Materials
The following supporting information can be downloaded online. 1H-NMR, 13C-NMR, Mass, and IR spectra of compound 3.
Author Contributions
Conceptualization, Y.-H.S.; methodology, X.Y. and Y.-H.S.; investigation, X.Y.; writing—original draft preparation, Y.-H.S.; writing—review and editing, Y.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Mokwon University.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Cheke, R.S.; Patel, H.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; Pasupuleti, V.R.; et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 2022, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Balappa Somappa, S. Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure-activity relationship. J. Clin. Pharm. Ther. 2022, 47, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer potentials of coumarin and its derivatives. Mini Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, D.; Liu, Y.; Li, M. Pharmacological perspectives and molecular mechanisms of coumarin derivatives against virus disease. Genes Dis. 2021, 9, 80–94. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.Z.; Zhao, Y.L.; Fan, D.W.; Xia, Y.J.; Zhang, P. Synthesis, crystal structure, photo- and electro-luminescence of 3-(4-(anthracen-10-yl)phenyl)-7-(N,N’-diethylamino)coumarin. Synth. Met. 2010, 160, 1642–1647. [Google Scholar] [CrossRef]
- Voutsadaki, S.; Tsikalas, G.K.; Klontzas, E.; Froudakis, G.E.; Katerinopoulos, H.E. A “turn-on” coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media. Chem. Commun. 2010, 46, 3292–3294. [Google Scholar] [CrossRef] [PubMed]
- Barghash, R.F.; Eldehna, W.M.; Kovalová, M.; Vojáčkva, V.; Kryštof, V.; Abdel-Aziz, H.A. One-pot three-component synthesis of novel pyrazolo[3,4-b]pyridines as potent antileukemic agents. Eur. J. Med. Chem. 2022, 227, 113952. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Mahajan, T.R.; Gole, Y.R.; Nambiar, M.; Matan, T.T.; Kulkarni-Almeida, A.; Balachandran, S.; Junjappa, H.; Balakrishnan, A.; Vishwakarma, R.A. Synthesis and evaluation of pyrazolo[3,4-b]pyridines and its structural analogues as TNF-α and IL-6 inhibitors. Bioorganic Med. Chem. 2008, 16, 7167–7176. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Júnior, N.M.; Mendes, T.C.F.; Leal, D.M.; Corrêa, C.M.N.; Sudo, R.T.; Zapata-Sudo, G.; Bareiro, E.J.; Fraga, C.A.M. Microwave-assisted synthesis and structure-activity relationships of neuroactive pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine derivative. Bioorganic Med. Chem. Lett. 2010, 20, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Mac, M.; Uchacz, T.; Danel, A.; Danel, K.; Kolek, P.; Kulig, E. A new fluorescent sensor based on 1H-pyrazolo[3,4-b]quinoline skeleton. J. Fluoresc. 2011, 21, 375–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.-H. Thieno[2,3-d]pyrimidines are a promising scaffold in medicinal chemistry: Recent advances. Bioorganic Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Singh, H.; Raj, T.; Sharma, A.; Singh, G.; Bariwal, J. 4-Substituted thieno[2,3-d]pyrimidines as potent antibacterial agents: Rational dssign, microwave-assisted synthesis, biological evaluation and molecular docking studies. Chem. Biol. Drug Des. 2017, 90, 1115–1121. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Yang, J.; Zaho, H.; Deng, B.; Ren, Y.; Mai, R.; Huang, J.; Chen, J. Discovery of thieno[2,3-d]pyrimidine-based KRAS G12D inhibitors as potential anticancer agents via combinatorial virtual screening. Eur. J. Med. Chem. 2022, 233, 114243. [Google Scholar] [CrossRef]
- Barrows, R.D.; Hammill, J.T.; Tran, M.C.; Falade, M.O.; Rice, A.L.; Davis, C.W.; Emge, T.J.; Rablen, P.R.; Kiplin Guy, R.; Knapp, S. Evaluation of 1,1-cyclopropylidene as a thioether isostere in the 4-thio-thienopyrimidine (TTP) series of antimalarials. Bioorganic Med. Chem. 2020, 28, 115758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, L.; Han, C.; Lv, H.; Chen, D.; Shen, G.; Wu, K.; Pan, S.; Ye, F. Design, synthesis, and biological activity tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivatives an anit-inflammatory agents. Molecules 2017, 22, 1960. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kim, S.M.; Rho, M.C.; Lee, S.W.; Song, Y.-H. Synthesis of thienopyrimidine derivatives as inhibitors of STAT3 activation induced by IL-6. J. Microbiol. Biotechnol. 2019, 29, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.; Ma, J.; Xu, H.; Wu, J.; Tang, X.; Fan, Z.; Wang, P. Synthesis and properties of fluorescence dyes: Tetracyclic pyrazolo[3,4-b]pyridine-based coumarin chromophores with intramolecular charge transfer character. J. Org. Chem. 2012, 77, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-C.; Lin, W.; Wang, H.-Y.; Hung, Z.-B.; Shi, D.-Q. Improved and efficient synthesis of chromeno[4,3-d]pyrazolo[3,4-b]pyridine-6(3H)-ones and their fluorescence properties. J. Heterocyclic Chem. 2014, 51, 1036–1044. [Google Scholar] [CrossRef]
- Park, J.W.; Song, Y.-H. Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids. Heterocycl. Commun. 2017, 23, 281–285. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bayat, Y.; Habibi, D.; Moshaee, S. FeCl3-SiO2 as a reusable heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles via [2+3] cycloaddition of nitriles and sodium azide. Tetrahedron Lett. 2009, 50, 4435–4438. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).