Abstract
A binuclear gadolinium (III) complex was obtained through the interaction of GdCl3·6H2O, 4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dione and NaOH in MeOH solution. Molar ration of reagents equal to 1:3:3 was used. Upon recrystallization of wet 1,4-dioxane, an intermediate hydrated complex formed stable crystalline solvate with the composition [(Gd2(L)3(H2O))2(C4H8O2)]•2(C4H8O2). The structured of complex was established by single crystal XRD experiment. Furthermore, some photophysical properties of the complex were measured. Thus, the energy of the first exited triplet state for the diketonate ligand was found to be 22400 cm−1, which makes 4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dione a promising ligand for the preparation of highly luminescent Eu3+ and Sm3+ complexes.
1. Introduction
Pyrazole substituted 1,3-diketones are a new and promising class of heterocyclic 1,3-diketones with isolated pyrazole moiety [1,2,3]. Due to the presence of additional coordination sites in the molecule (“pyridine-like” N(2) atom of pyrazole fragment), they may be interesting as ligands for the construction of supramolecular ensembles such as coordination polymers of different degree of dimensionality [4,5,6]. Usually, most of efforts are focused on synthesis of complexes with higly-luminescent lanthanide ions such as Eu3+, Tb3+, Sm3+ or Yb3+ [7,8,9], but role of non-luminescent Gd3+ complexes are also very important. Since the energy of resonant 6P7/2 level in Gd3+ ion is much higher (~31,000 cm−1) than the energy of the first triplet level (T1) of most organic ligands, no energy transfer between ligand and Gd3+ is possible; instead, only phosphorescence of ligands can be observed especially under low (77 K) temperatures. Consequently, Gd3+ complexes are a useful tool for the determination of T1 energy levels in coordination compounds [10]. Recently, we have introduced [6] 4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dione (HL, 1) as a new ligand for the coordination chemistry of lanthanides, but simple hydrated complex [Gd(L)3(H2O)2] was not fully characterized up to now. Here, we wish to report on synthesis and the crystal structure of more a complex molecule—[(Gd2(L)3(H2O))2(C4H8O2)]•2(C4H8O2), where C4H8O2 is 1,4-dioxane. This complex is pretty stable and can be isolated in pure crystalline form.
2. Results and Discussion
Preparation of the complex was performed in the usual manner by the interaction of hydrated GdCl3, ligand 1 and a stoichiometric amount of NaOH in MeOH solution (Scheme 1). Since hydrated pyrazolic complexes usually fail to crystallize from the solution, the reaction mixture was slowly evaporated to a small volume and the complex was precipitated by the addition of an equal volume of distilled water [11].
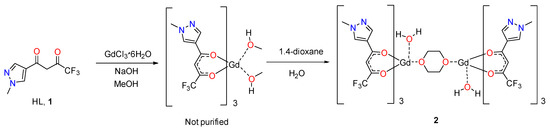
Scheme 1.
Synthesis of complex 2.
According to the data of the elemental analysis, the composition of the amorphous precipitate is closed to [Gd(L)3(МеОН)2], but it was not possible to obtain crystals by recrystallization from MeOH or other alcohols (EtOH, i-PrOH and their mixtures). The crystallization from 1,4-dioxane was found to be the most effective—an amorphous powder was dissolved in a minimum amount of wet dioxane, filtered and the saturated solution was left at room temperature for slow evaporation of the solvent. A copious crystalline powder of complex 2 was obtained after several days. The structure of dried powder was established on the basis of an elemental analysis and, additionally, by single crystal X-ray data. The obtained crystals are stable in air but lose solvent slowly with degradation upon gentle heating (60 °C) in a moderate vacuum (0.1 torr).
The IR spectrum (Figures S1 and S2, SI) shows bands corresponding to vibrations of OH groups involved in H-bonds system (3132 cm−1). The series of bands at 2969, 2768 and 2673 cm−1 refers to different C-H stretching vibrations of methyl groups and dioxane molecules. The C=O bands of the carbonyl groups appear at 1684 and 1618 cm−1, and the shift relative to the free ligand confirms the coordination and formation of the chelate ring. Vibrations of the pyrazole ring, as well as vibrations of C-F bonds, appear in the region of 1400–1100 cm−1 and partially overlap each other.
Single crystals of 2 suitable for X-ray crystallography were obtained by crystallization from wet dioxane. The asymmetric unit of the crystal consists of a fragment [Gd(L)3(H2O)(C4H8O2)0.5], alongside one solvated molecule of dioxane (Figure 1), leading to a binuclear complex [Gd2(L)6(H2O)2•2(C4H8O2). Thus, 2 is present in the crystal as centrosymmetric dimeric molecules with one dioxane molecule acting as a bridging ligand, as shown in the Figure 2.
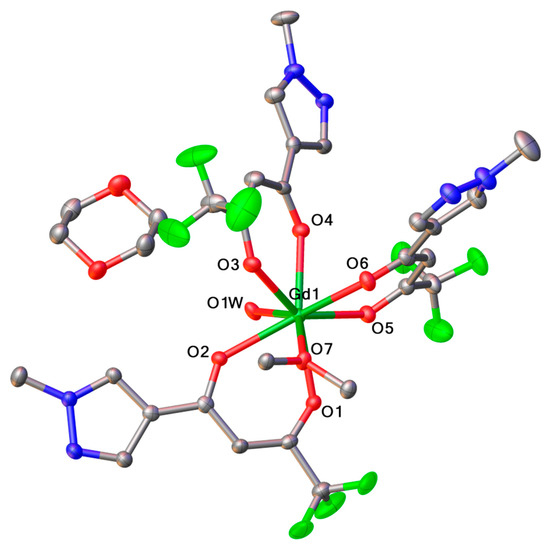
Figure 1.
View of the asymmetric unit of 2 crystal. Thermal ellipsoids are presented at 50% probability, and hydrogen atoms are omitted for clarity.
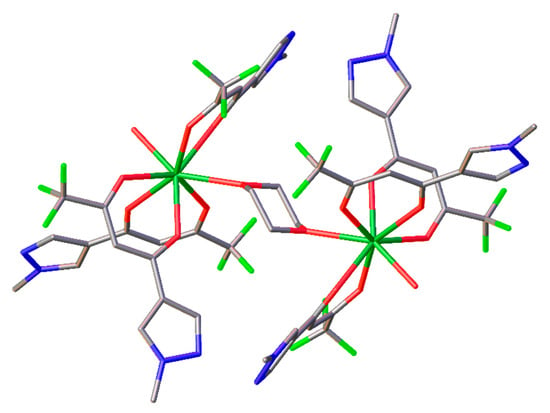
Figure 2.
View of the 2 molecule. Dioxane molecules (solvated), hydrogen atoms and thermal ellipsoids are omitted for clarity.
Gd3+ ion is coordinated by six oxygen atoms of three diketonate ligands, 1 oxygen atom of water molecule and 1 oxygen atom of bridging dioxane ligand, which results in an octa-coordinated lanthanide ion. According to the results of the SHAPE analysis [12], the most suitable coordination polyhedron for the central metal ion is square antiprism with basal planes O1O5O6O7 and O1WO2O3O4 (Figure 3, Table 1). These two faces of the polygon are nearly parallel (angle between the basal planes is 6.2°) and the distance between them is 2.577 Å. According to the Table 2 that contains Gd-O bond lengths, mean Gd-OL distance is 2.36 Å, which is in accordance with that obtained from the CCDC analysis (2.37 Å).
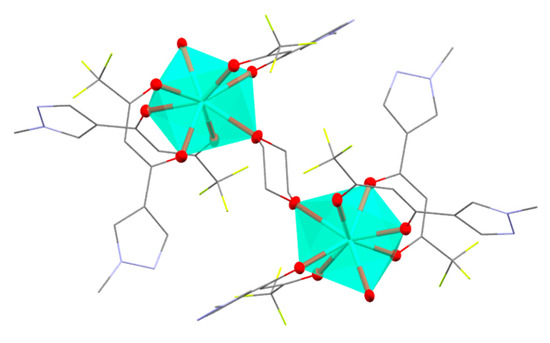
Figure 3.
View of Gd3+ polyhedron in 2. Dioxane molecules (solvated), hydrogen atoms and thermal ellipsoids of atoms not coordinated to Gd3+ are omitted for clarity.

Table 1.
Results of the SHAPE analysis of Gd3+ polyhedron in 2.

Table 2.
Selected geometrical parameters for 2.
The analysis of crystal packing of 2 reveals the presence of hydrogen bonds O1W-H…N (O1W…N1 distance is 2.80 Å, O1W…N3 distance is 2.79 Å) leading to the 2 packing in the form of linear polymeric chains, as illustrated in Figure 4.
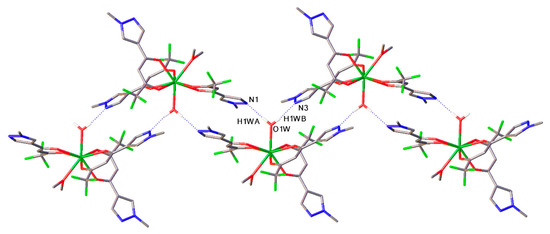
Figure 4.
Fragment of crystal packing of 2. Hydrogen O-H…N bonds are presented as dotted lines.
The identity of structure for single crystal and bulk material was confirmed by powder XRD (Figure S3, SI). The experimental pattern, obtained for powdered bulk material, is close to those simulated from single-crystal diffraction data. Small deviations between simulated and experimental patterns may be attributed to effects of the texture of the powdered sample. Additionally, it was show, that bulk material is pure single-phase.
The UV absorption spectra for HL (1) and complex 2 were recorded in an MeCN solution at an ambient temperature (Figure 5). They all exhibit the spin-allowed broad bands corresponding to the π → π* transitions in the ligands. The observed maxima at 259 and 314 nm nm can be assigned to the absorption of pyrazole aromatic system and conjugated diketone fragment, respectively. Based on the spectral data, one can conclude that the chemical bonding of ligands with Gd3+ ion lead to a significant enhancement of the molar extinction. In contrast to the weakly absorbing hydrated Gd3+ ion (ε~10 M−1 cm−1), the molar absorption coefficients for the complexes are approximately in 103 times higher and reach 2–6 × 104 M−1cm−1.
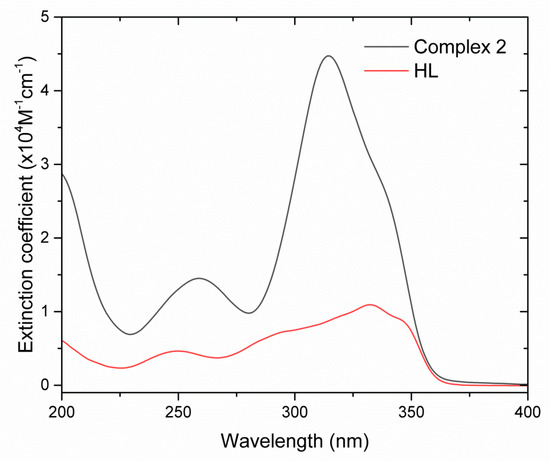
Figure 5.
Absorption spectra of ligand 1 and complex 2 in MeCN solutions.
The energy of first triplet level of the ligand 1 was determined from the phosphorescent spectrum of 2 at 77 K. Since the energies of S1 and T1 levels of aliphatic molecules are much higher than for aromatic one, for the complex 2, the T1 level (the lowest one) de facto is associated only with diketonate fragment of the binuclear complex. Other ligands such as H2O or dioxane do not interfere with the determination of the triplet level energy from the phosphorescence spectrum [11]. The tangent method [10] was used to determine the T1 energy level and it was found to be 22,400 cm−1 (Figure 6).
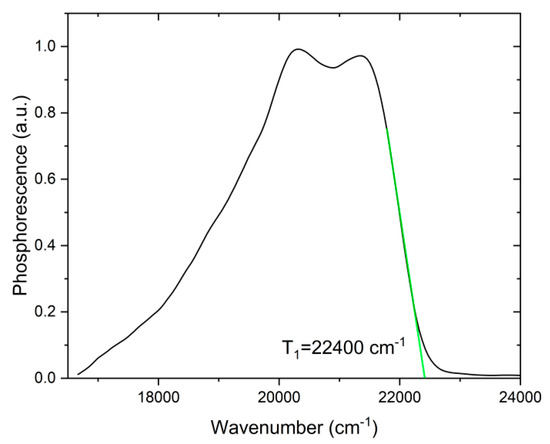
Figure 6.
Low-temperature (77 K) phosphorescent spectrum of complex 2 in solid form.
The T1 energy level value of 22,400 cm−1 is favorable for sensibilization of Eu3+ and Sm3+ ions, because the energy gaps between resonant levels of these ions and T1 level of ligands lay in an optimum range of 2000–3000 cm−1 according to the empirical Latva’s rule [10]. One may expect that corresponding complexes may be an efficient luminescent materials.
3. Materials and Methods
All reagents were purchased form Aldrich (St. Louis, MO, USA) and were used without further purification. Ligand - 4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dione (1) was synthesized according previously published procedure [13]. Elemental analysis was performed by Laboratory of Microanalysis of Nesmeyanov Institute of Organoelement compounds (Moscow, Russian Federation). FTIR spectra were recorded in KBr pellets on Perkin Elmer Spectrum One instrument (Santa Barbara, CA, USA). Single-crystal X-ray diffraction analysis was carried out on a Bruker D8 Quest diffractometer (Bruker AXS Handheld Inc., Kennewick, WA, USA), MoKα radiation, ω and φ-scan mode. Single X-ray diffraction analysis was carried out on a Bruker D8 Quest diffractometer (MoKα radiation, ω and φ-scan mode). The structure was solved with direct methods and refined by least-squares method in the full-matrix anisotropic approximation on F2. All hydrogen atoms were located in geometric positions and refined freely. All calculations were performed using the SHELXTL [14,15] and Olex2 [16] software packages. Crystal Data for C60H64F18Gd2N12O20 (M = 1929.73 g/mol): triclinic, space group P-1 (no. 2), a = 13.3891(7) Å, b = 13.5291(7) Å, c = 16.1602(7) Å, α = 86.016(2)°, β = 66.320(2)°, γ = 62.099(2)°, V = 2343.7(2) Å3, Z = 1, T = 110(2) K, μ(MoKα) = 1.499 mm-1, Dcalc = 1.367 g/cm3, 23351 reflections measured (2.78° ≤ 2Θ ≤ 58.00°), 12294 unique (Rint = 0.0564, Rsigma = 0.0910) which were used in all calculations. The final R1 was 0.0446 (I > 2σ(I)) and wR2 was 0.0974 (all data). Atomic coordinates, bond lengths, angles, and thermal parameters have been deposited at the Cambridge Crystallographic Data Centre with deposition number CCDC 2201754, which is available free of charge at www.ccdc.cam.ac.uk.
Powder diffractograms were recorded using a diffractometer of the BrukerD8 Advance (CuKα, λ = 1.5418 Å). Photoluminescence spectra and lifetimes of excited states of powdered samples were measured using a Fluorolog-QM (Horiba, Kioto, Japan) spectrofluorimeter equipped with a 75 W ArcTune xenon lamp and a Hamamatsu R13456 photomultiplier sensitive in the radiation range of 200–980 nm. For low-temperature measurements, the sample was immersed in a quartz optical cryostat filled by liquid nitrogen. Absorption spectra were measured on a Jasco V-770 instrument (Jasco, Tokyo, Japan) in 1 cm pathway quartz optical cells in the range of 250–900 nm.
Synthesis of (Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane, Complex 2
To a magnetically stirred solution of 0.246 g (1.2 mmol) of ligand HL (1), in 10 ml of MeOH at 45 °C 1M solution of NaOH in MeOH (1.2 mL, 1.2mmol) was added dropwise, and the resultant clear solution was stirred in a closed vial for 10 min. Then solution of GdCl3·6H2O (0.148 g, 0.4 mmol) in 2 mL of hot MeOH was slowly added, and the resultant mixture was incubated at 45 °C for 3 h in a closed vial. The mixture was cooled to a room temperature and left in an open vial until approximately 2/3 of solvent by volume evaporated slowly. Deionized water (3 mL) was added dropwise to a resulted slurry with vigorous magnetic stirring and the precipitate was filtered off and washed successively by 3 mL of 50% aqueous MeOH, 5 mL of deionized water and 5 mL of hexahe and dried in air to a constant weight. The crude product (0.227 g) was dissolved in 1,4-dioxane (5 mL), centrifugated at 11,000 rpm for 5 min and clear supernatant was left in a open beaker in air until crystallization occurred. Crystals were collected, washed with a few drops of cold 1,4-dioxane and dried in air to a constant weight. The yield was 0.386 g (43%).
Anal. calcd. for C60H64F18Gd2N12O20 (1929.69): C, 37.34; H, 3.34; N, 8.17; Gd, 16.30%. Found: C, 37.49; H, 3.27; N, 8.24; Gd, 16.41%. IR spectrum, ν, cm–1: 3600–3200 (wide, m); 3132 (vw); 2960 (vw); 2768 (vw); 2673 (vw); 1684 (vs); 1618 (vs); 1559 (s); 1523 (w); 1452 (m); 1390 (w); 1362 (vw); 1295 (vw); 1280 (vs); 1208 (vs); 1139 (s); 1087 (vw); 1069 (w); 993 (s); 950 (vw); 935 (w); 878 (w); 841 (w); 797 (s); 786 (vw); 721 (m); 711 (m); 665 (m); 651 (vw); 585 (w); 520 (vw); 456 (vw).
Supplementary Materials
The following are available online, Figure S1: FTIR spectrum of complex 2 in KBr pellet, Figure S2: FTIR spectrum of complex 2 in KBr pellet (extended region 2000–400 cm−1), Figure S3: Experimental and simulated from single-crystal data XPD patterns for complex 2.
Author Contributions
I.V.T.: project administration, funding acquisition, supervision, writing—original draft preparation; M.T.M.: investigation; V.E.G.: investigation, writing—original draft preparation; Y.A.B.: investigation, writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 19-13-00272.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge support from Lomonosov Moscow State University Program of Development for providing access to single X-ray diffraction equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Metlina, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Taydakov, I.V.; Lyssenko, K.A.; Vitukhnovsky, A.G.; Selyukov, A.S.; Krivobok, V.S.; Aminev, D.F.; Tobokhova, A.S. Luminescence and Electronic Structure of Nd3+ Complex with Pyrazole-Substituted 1,3-Diketone and 1,10-Phenanthroline. J. Lumin. 2018, 203, 546–553. [Google Scholar] [CrossRef]
- Sizov, V.S.; Komissar, D.A.; Metlina, D.A.; Aminev, D.F.; Ambrozevich, S.A.; Nefedov, S.E.; Varaksina, E.A.; Metlin, M.T.; Mislavskií, V.V.; Taydakov, I.V. Effect of Ancillary Ligands on Visible and NIR Luminescence of Sm3+ β-Diketonate Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117503. [Google Scholar] [CrossRef] [PubMed]
- Metlina, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Selyukov, A.S.; Datskevich, N.P.; Aminev, D.F.; Goryachii, D.O.; Lyssenko, K.A.; Pavlov, A.A.; Dmitrienko, A.O.; et al. Bright NIR-Luminescent Nd3+ Complexes with Pyrazole-Substituted 1,3-Diketones Demonstrated an Unusual Spectral Lines Branching Ratios. Dye. Pigment. 2020, 181, 108558. [Google Scholar] [CrossRef]
- Taidakov, I.; Krasnosel’skii, S.; Lobanov, A.; Vitukhnovskii, A.; Starikova, Z. Cesium Tetrakis(1-(1,5-Dimethyl-1H-Pyrazol-4-Yl)-4,4,4-Trifluorobutane-1,3-Diono)Europiate(III): Synthesis, Crystal Structure, and Luminescence Properties. Russ. J. Coord. Chem. 2013, 39, 680–684. [Google Scholar] [CrossRef]
- Taidakov, I.; Lobanov, A.; Vitukhnovskii, A.; Starikova, Z. Synthesis and Unusual Crystal Structure of the Eu(III) Complex with 1-(1,5-Dimethyl-1H-Pyrazol-4-Yl)-4,4,4-Trifluorobutane-1,3-Dione. Russ. J. Coord. Chem. 2013, 39, 437–441. [Google Scholar] [CrossRef]
- Gontcharenko, V.; Kiskin, M.; Dolzhenko, V.; Korshunov, V.; Taydakov, I.; Belousov, Y. Mono- and Mixed Metal Complexes of Eu3+, Gd3+, and Tb3+ with a Diketone, Bearing Pyrazole Moiety and CHF2-Group: Structure, Color Tuning, and Kinetics of Energy Transfer between Lanthanide Ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef] [PubMed]
- Trannoy, V.; Carneiro Neto, A.N.; Brites, C.D.S.; Carlos, L.D.; Serier-Brault, H. Engineering of Mixed Eu3+/Tb3+ Metal-Organic Frameworks Luminescent Thermometers with Tunable Sensitivity. Adv. Opt. Mater. 2021, 9, 2001938. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Rising Stars in Science and Technology: Luminescent Lanthanide Materials: Rising Stars in Science and Technology: Luminescent Lanthanide Materials. Eur. J. Inorg. Chem. 2017, 2017, 5058–5063. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide Azolecarboxylate Compounds: Structure, Luminescent Properties and Applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthanide Luminescence; Hänninen, P., Härmä, H., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2010; Volume 7, pp. 1–45. ISBN 978-3-642-21022-8. [Google Scholar]
- Metlin, M.T.; Goryachii, D.O.; Aminev, D.F.; Datskevich, N.P.; Korshunov, V.M.; Metlina, D.A.; Pavlov, A.A.; Mikhalchenko, L.V.; Kiskin, M.A.; Garaeva, V.V.; et al. Bright Yb3+ Complexes for Efficient Pure Near-Infrared OLEDs. Dye. Pigment. 2021, 195, 109701. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Taydakov, I.V.; Krasnoselsky, S.S. Modified Method for the Synthesis of Isomeric N-Substituted (1H-Pyrazolyl)Propane-1,3-Diones. Chem. Heterocycl. Compd. 2011, 47, 695. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).