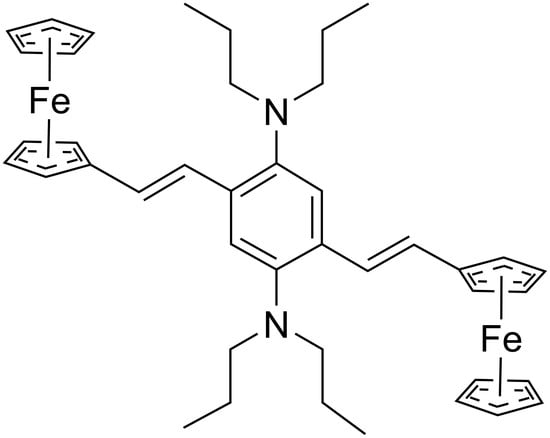
2,5-Bis((E)-2-ferrocenylvinyl)-N,N,N′,N′-tetrapropylbenzene-1,4-diamine
Abstract
:1. Introduction
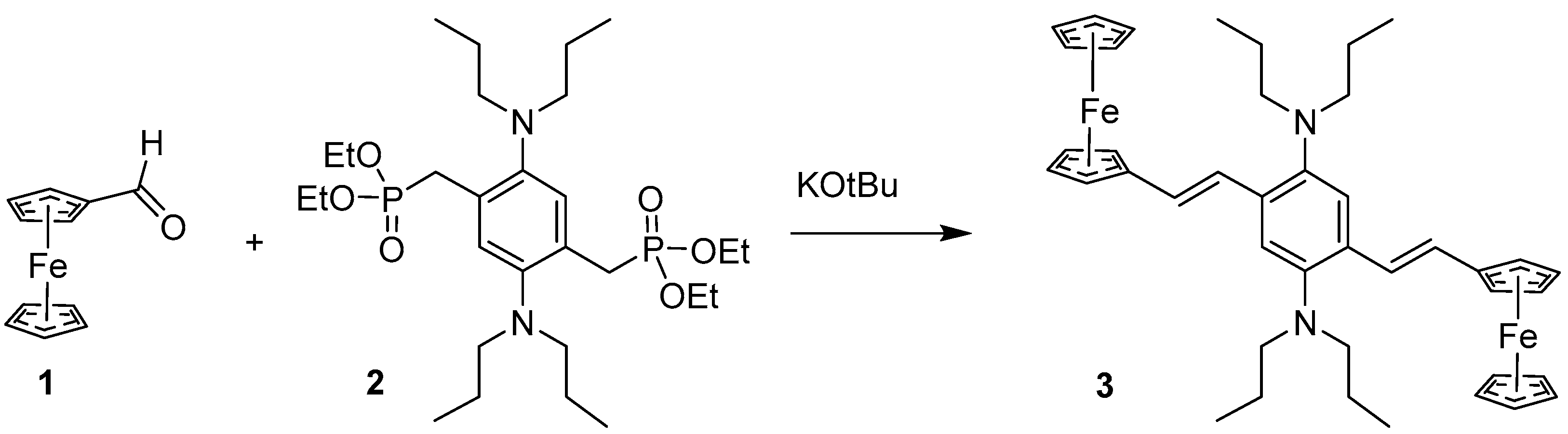
2. Synthesis
3. Optical Spectroscopy
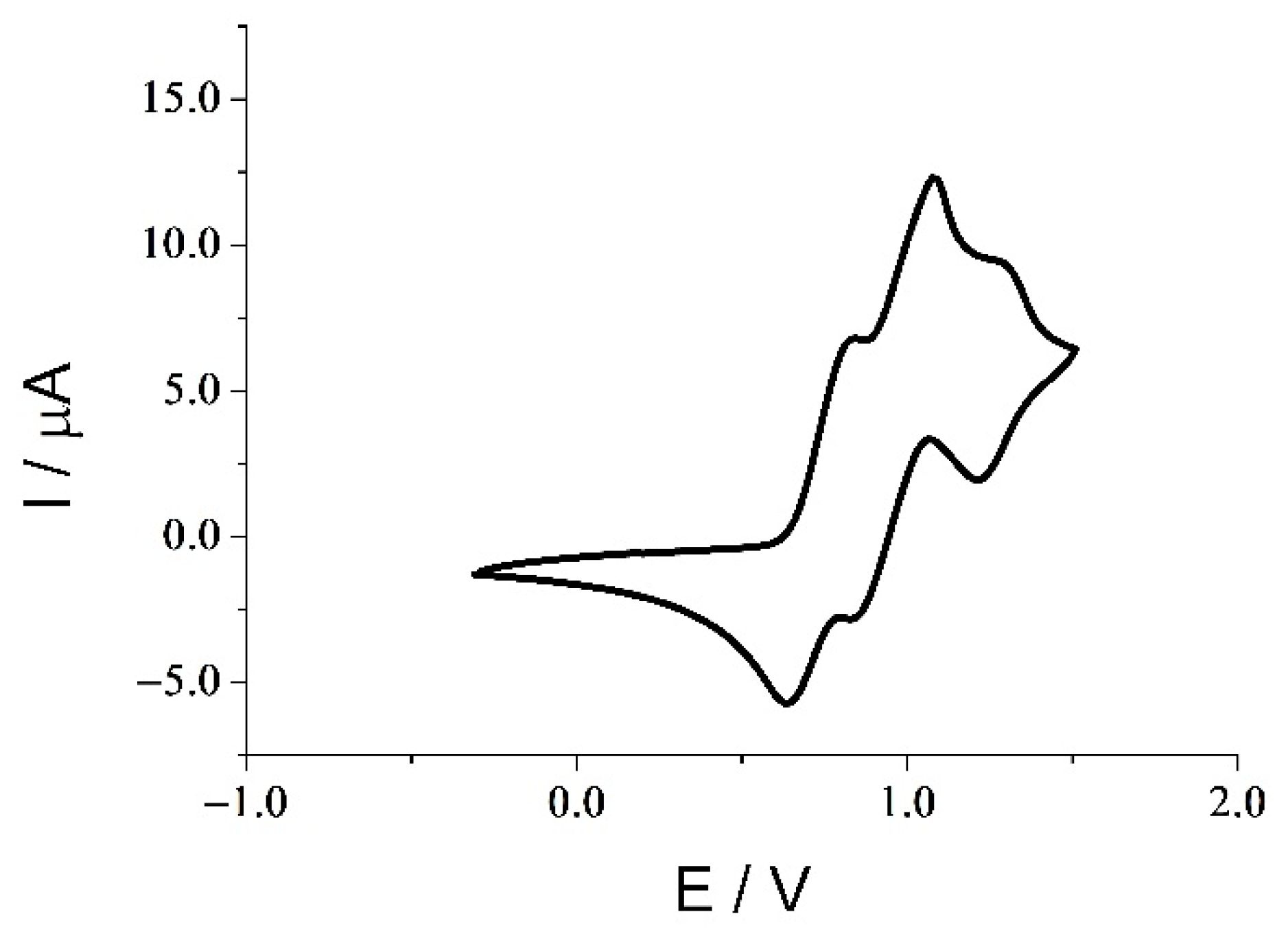
4. Electrochemistry
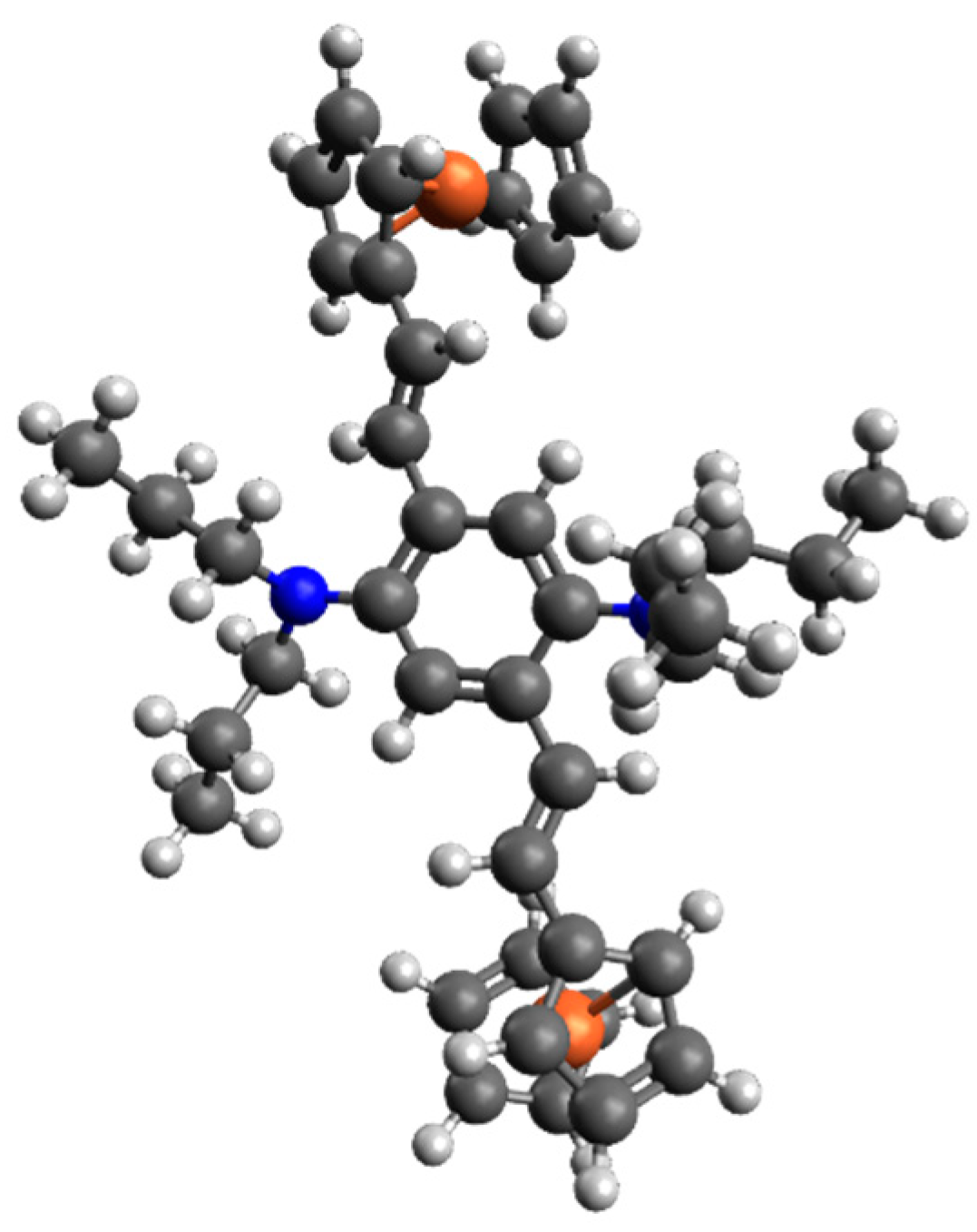
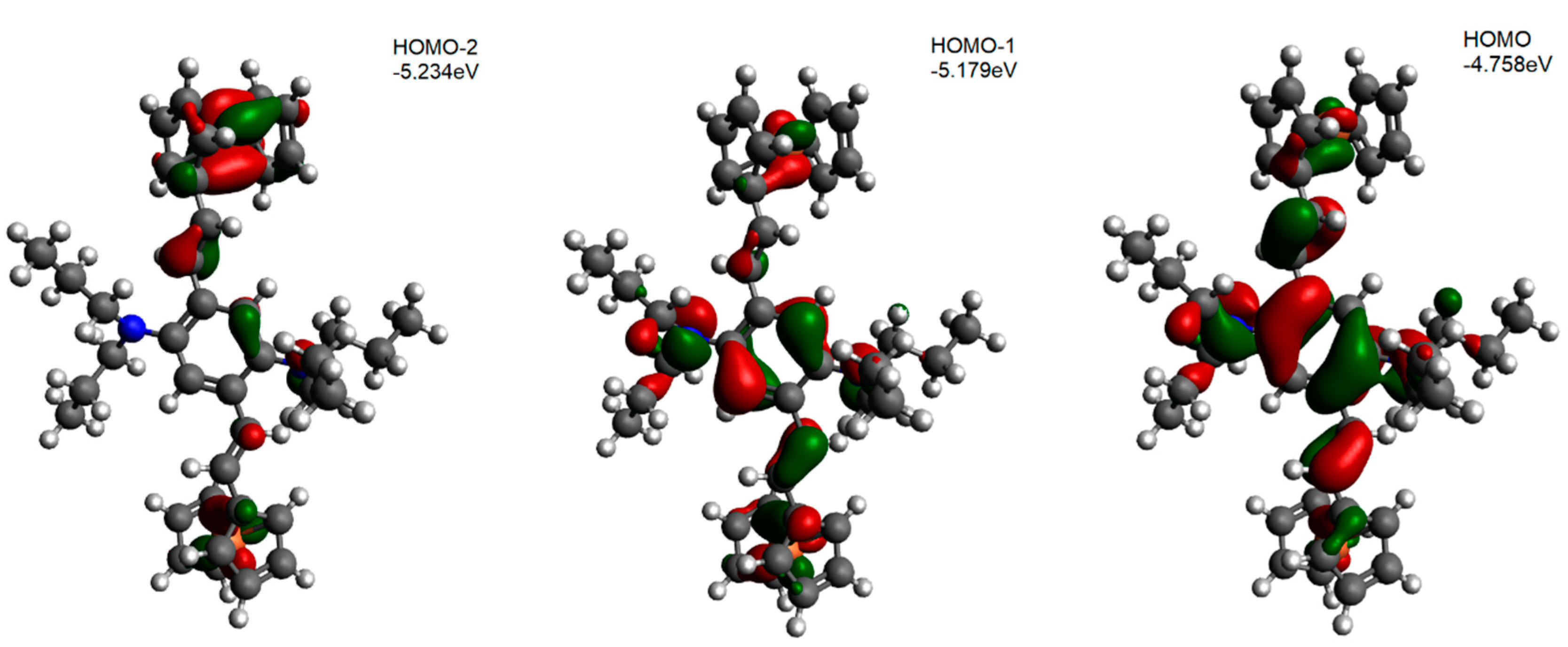
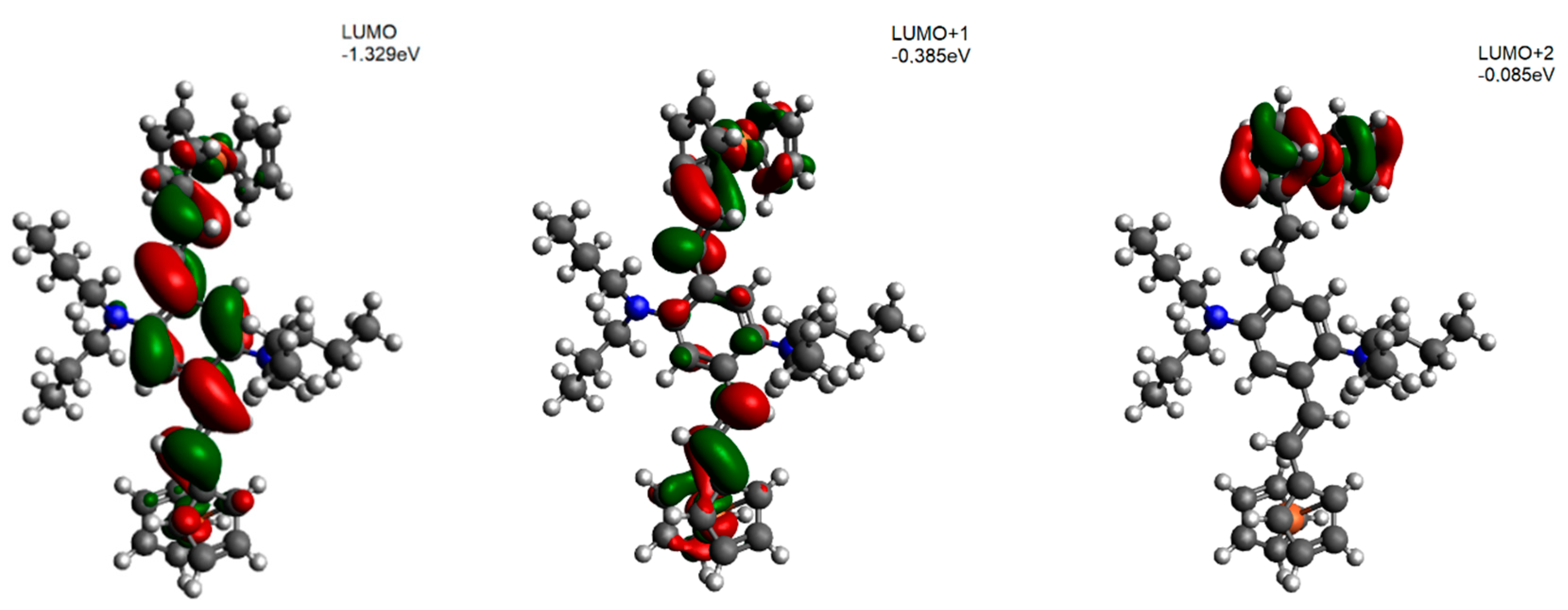
5. DFT Calculation Mechanics
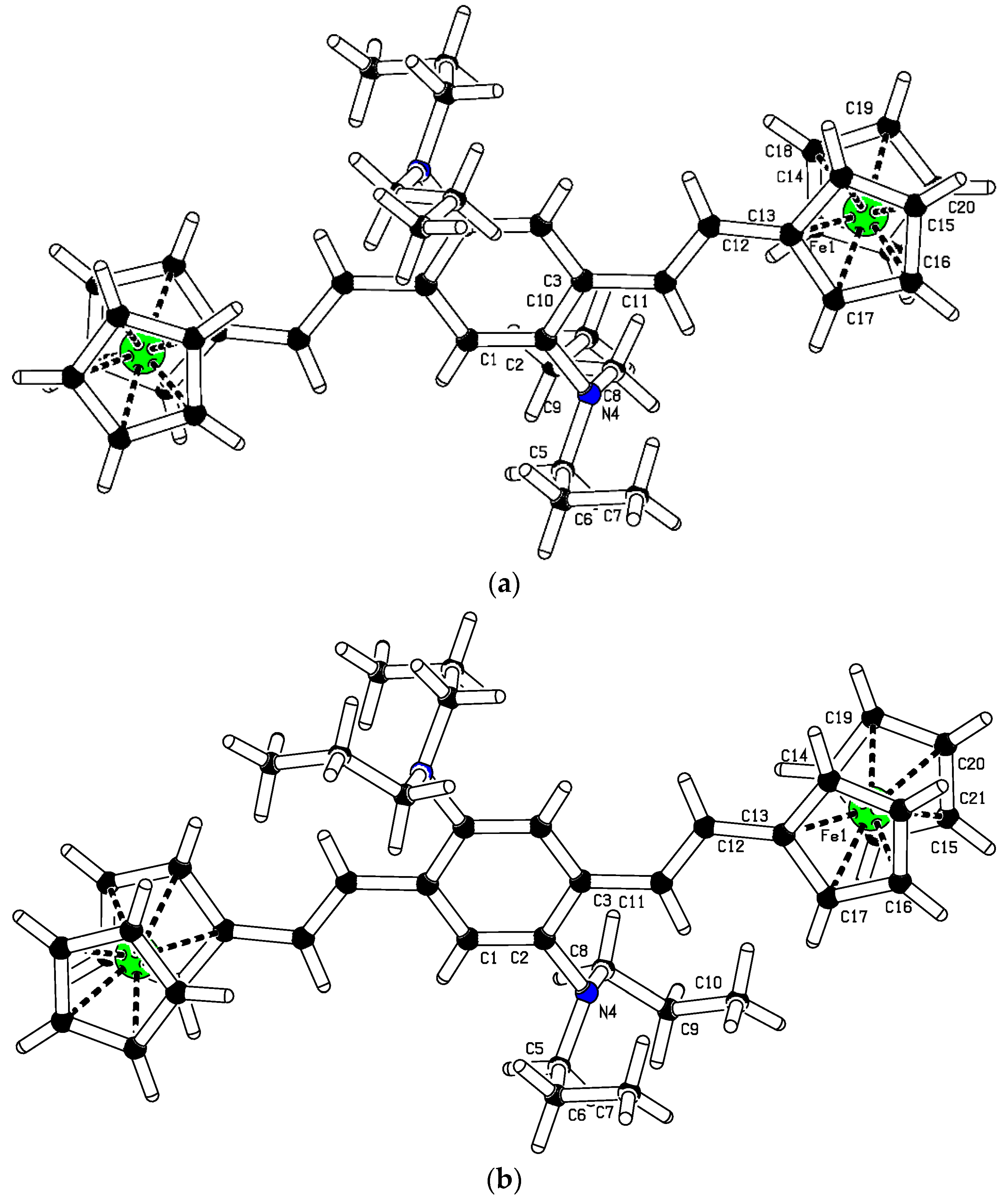
6. Crystal Structure
7. Discussion
8. Conclusions
9. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar]
- Kanibolotsky, A.L.; Perepichka, I.F.; Skabara, P.J. Star-shaped π-conjugated oligomers and their applications in organic electronics and photonics. Chem. Soc. Rev. 2010, 39, 2695–2728. [Google Scholar]
- Tolosa, J.; Zucchero, A.J.; Bunz, U.H.F. Water-soluble cruciforms: Response to protons and selected metal ions. J. Am. Chem. Soc. 2008, 130, 6498–6506. [Google Scholar]
- Schmitt, V.; Moschel, S.; Detert, H. Diaryldistyrylpyrazines: Solvatochromic and Acidochromic Fluorophores. Eur. J. Org. Chem. 2013, 25, 5655–5669. [Google Scholar]
- Lehmann, M.; Dechant, M.; Hügel, M.; Scheuring, N.; Ghosh, T. Fullerene-Filled Stilbene Stars: The Balance between Isolated C60 Helices and 3D Networks in Liquid Crystal Self-Assemblies. Chem. Eur. J. 2019, 58, 3352–3361. [Google Scholar]
- Maier, P.; Grüne, M.; Lehmann, M. A Star-shaped Oligo(phenylenevinylene) Liquid Crystal Host with an Anthracene Guest—A Double Nanosegregating Supermesogen. Chem. Eur. J. 2017, 23, 1018–1022. [Google Scholar]
- Kundu, R.; De, S. Push-pull unit decorated oligomer for organic electronics. Adv. Chem. Res. 2019, 53, 21–68. [Google Scholar]
- Patra, A.; Bendikov, M. Selenium- and tellurium-containing organic π-conjugated oligomers and polymers. In Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Rappoport, Z., Eds.; Wiley: Hoboken, NJ, USA, 2012; Volume 3, pp. 523–583. [Google Scholar]
- Hissler, M.; Dyer, P.W.; Reau, R. Linear organic π-conjugated systems featuring the heavy Group 14 and 15 elements. Chem. Rev. 2003, 244, 1–44. [Google Scholar]
- Liu, Y.; Li, Y.; Schanze, K.S. Photophysics of π-conjugated oligomers and polymers that contain transition metal complexes. J. Photochem. Photobiol. C 2002, 3, 1–23. [Google Scholar]
- Meng, Z.; Kan, S.; Sukegawa, T.; Oyaizu, K.; Ho, C.-L.; Xiang, J.; Feng, Y.-H.; Lo, Y.H.; Nishide, H.; Wong, W.-Y. Metallopolyyne polymers with ferrocenyl pendant ligands as cathode-active materials for organic battery application. J. Organomet. Chem. 2016, 812, 51–55. [Google Scholar]
- Peris, E. From long-chain conjugated oligomers to dendrimers: Synthesis and physical properties of phenyl-ethenyl-ferrocenyl containing one- and two-dimensional complexes. Coord. Chem. Rev. 2004, 248, 279–297. [Google Scholar]
- Heinze, K.; Lang, H. Ferrocene-Beauty and Function. Organometallics 2013, 32, 5623–5625. [Google Scholar]
- Plenio, H.; Hermann, J.; Sehring, A. Optically and redox-active ferrocene-acetylene polymers and oligomers. Chem. Eur. J. 2000, 6, 1820–1829. [Google Scholar]
- Gao, X.; Deng, L.; Hu, J.; Zhang, H. Ferrocene-containing conjugated oligomers synthesized by acyclic diene metathesis polymerization. Polymers 2019, 11, 1334–1343. [Google Scholar]
- Yasuda, T.; Abe, J.; Iyoda, T.; Kawai, T. Selective synthesis of 1-aryl-2-ferrocenylethylene by cross-metathesis. Chem. Lett. 2001, 8, 812–813. [Google Scholar]
- Pauson, P.L.; Watts, E.E. Ferrocene derivatives. XIII. Some ferrocenylethylene and -acetylene derivatives. J. Chem. Soc 1963, 2990–2996. [Google Scholar] [CrossRef]
- Kournoutas, F.; Kalis, I.K.; Feckova, M.; Achelle, S.; Fakis, M. The effect of protonation on the excited state dynamics of pyrimidine chromophores. J. Photochem. Photobiol. A 2020, 391, 112398. [Google Scholar]
- Hoffert, K.; Durand, R.J.; Gauthier, S.; Robin-le Guen, F.; Achelle, S. Synthesis and Photophysical Properties of a Series of Pyrazine-Based Push-Pull Chromophores. Eur. J. Org. Chem. 2017, 3, 523–529. [Google Scholar]
- Zucchero, A.J.; McGrier, P.; Bunz, U.H.F. Cross-Conjugated Cruciform Fluorophores. Acc. Chem. Res. 2010, 43, 397–408. [Google Scholar]
- Achelle, S.; Rodruiguez-Lopez, J.; Robin-Le Guen, F. Photoluminescence Properties of Aryl-, Arylvinyl-, and Arylethynylpyrimidine Derivatives. ChemistrySelect 2018, 3, 1852–1886. [Google Scholar]
- Wink, C.; Detert, H. Donor-substituted Distyrylpyrazines: Influence of steric congestion on UV-vis absorption and fluorescence. J. Phys. Org. Chem. 2013, 26, 137–143. [Google Scholar]
- Horner, L.; Hoffman, H.; Wippel, H.G.; Klahre, G. Phosphorus organic compounds. XX. Phosphine oxides as reagents for olefin formation. Chem. Ber. 1959, 92, 2499–2505. [Google Scholar]
- Lifka, T.; Kalbitz, H.; Meier, H. Mesomorphic donor-acceptor-substituted 1,4-distyrylbenzenes. Z. Für Nat. B 2009, 64, 1183–1186. [Google Scholar]
- Meier, H.; Kim, S.; Oehlhof, A. Dendritic triphenylmethylium and tetraphenylmethane compounds. Synthesis 2009, 05, 848–852. [Google Scholar]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of Light-Emitting Conjugated Polymers for Applications in Electroluminescent Devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar]
- Schmitt, V.; Holzmann, G.; Schollmeyer, D.; Detert, H. [2,5-Bis(dipropylamino)-4-(hydroxymethyl)phenyl]methanol. IUCrData 2021, 6, x210443. [Google Scholar]
- Detert, H.; Sugiono, E. Bis(pyridylvinyl)diaminobenzenes: Synthesis, acidochromism and solvatochromism of the fluorescence. J. Luminesc. 2005, 112, 372–376. [Google Scholar]
- Schmitt, V.; Glang, S.; Preis, J.; Detert, H. Proton-induced multiple changes of the absorption and fluorescence spectra of amino-aza-oligo(phenylenevinylene)s. Sens. Lett. 2008, 6, 524–530. [Google Scholar]
- Armstrong, A.T.; Smith, F.; Elder, E.; McGlynn, E.P. Electronic Absorption Spectrum of Ferrocene. J. Chem. Phys. 1967, 46, 4321–4328. [Google Scholar]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar]
- Seiler, P.; Dunitz, J.D. A new interpretation of the disordered crystal structure of ferrocene. Acta Cryst. B 1979, 35, 1068–1074. [Google Scholar]
- Takusagawa, F.; Koetzle, T.F. A Neutron Diffraction Study of the Crystal Structure of Ferrocene. Acta Cryst. B 1979, 35, 1074–1081. [Google Scholar]
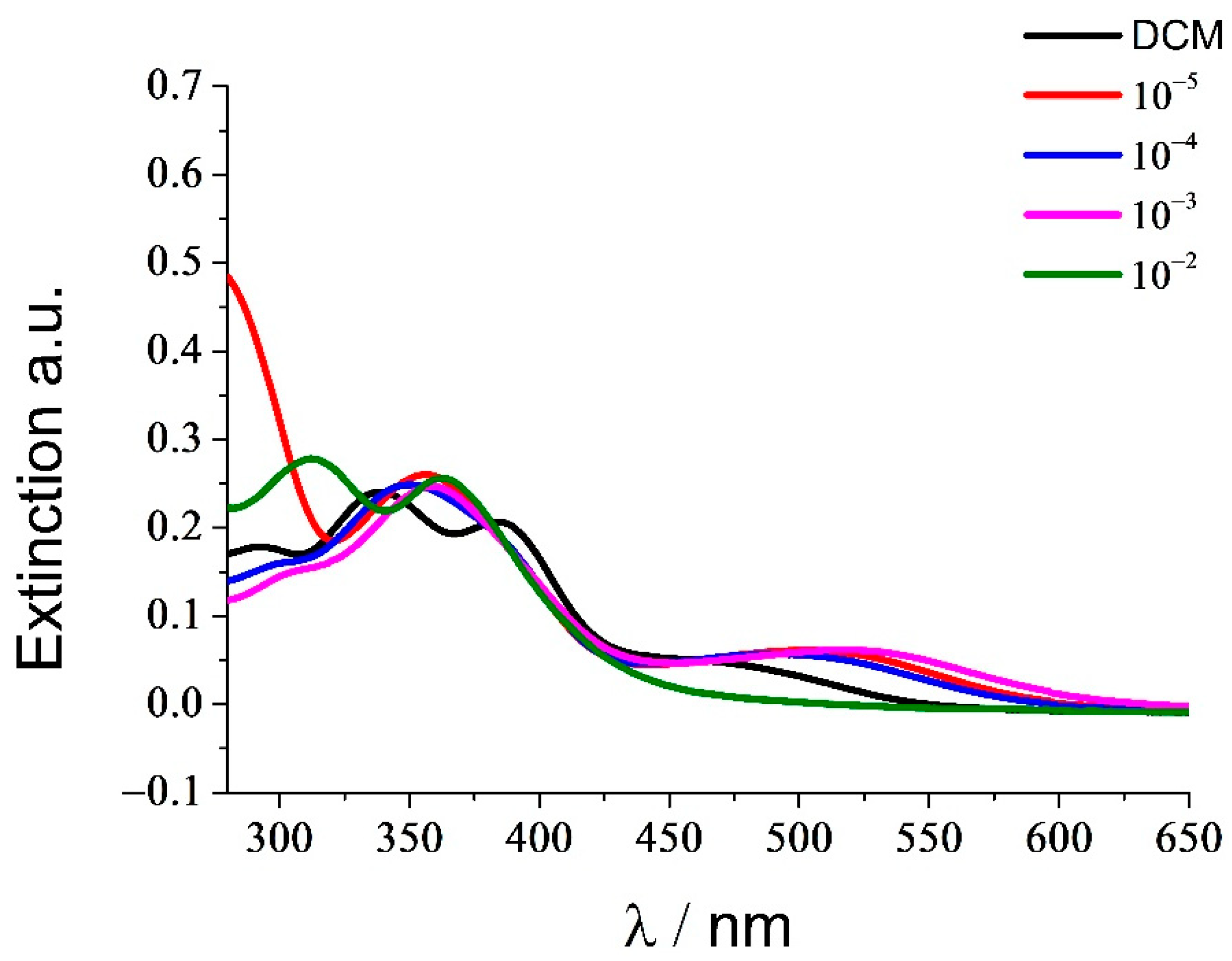
| Entry | TFA / | λ1 /nm | log ε1 | λ2 /nm | log ε2 | λ3 /nm | log ε3 |
|---|---|---|---|---|---|---|---|
| 1 | DCM | 455 | 4.71 | 385 | 5.31 | 339 | 5.38 |
| 2 | 10−5 | 505 | 4.79 | - | 355 | 5.41 | |
| 3 | 10−4 | 497 | 4.75 | - | 350 | 5.46 | |
| 4 | 10−3 | 515 | 4.79 | - | 358 | 5.39 | |
| 5 | 10−2 | - | 362 | 5.41 | 312 | 5.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochem, M.; Proz, I.; Schollmeyer, D.; Detert, H. 2,5-Bis((E)-2-ferrocenylvinyl)-N,N,N′,N′-tetrapropylbenzene-1,4-diamine. Molbank 2022, 2022, M1420. https://doi.org/10.3390/M1420
Jochem M, Proz I, Schollmeyer D, Detert H. 2,5-Bis((E)-2-ferrocenylvinyl)-N,N,N′,N′-tetrapropylbenzene-1,4-diamine. Molbank. 2022; 2022(3):M1420. https://doi.org/10.3390/M1420
Chicago/Turabian StyleJochem, Matthias, Igor Proz, Dieter Schollmeyer, and Heiner Detert. 2022. "2,5-Bis((E)-2-ferrocenylvinyl)-N,N,N′,N′-tetrapropylbenzene-1,4-diamine" Molbank 2022, no. 3: M1420. https://doi.org/10.3390/M1420
APA StyleJochem, M., Proz, I., Schollmeyer, D., & Detert, H. (2022). 2,5-Bis((E)-2-ferrocenylvinyl)-N,N,N′,N′-tetrapropylbenzene-1,4-diamine. Molbank, 2022(3), M1420. https://doi.org/10.3390/M1420