Abstract
Over the last decade, there has been an increasing effort to fight inflammatory conditions establishing new multitarget approaches. Chronic inflammation is implicated in many multifactorial diseases, constituting a great economic burden and a chronic health problem. In an attempt to develop new potent multifunctional anti-inflammatory agents, a cinnamic-pyrrole hybrid (6) was synthesized and screened for its antioxidant and anti-Lipoxygenase potential. The new compound, in comparison with its pyrrole precursor (4), showed improved biological activities. In silico calculations were performed to predict its drug-likeness. The examined derivative is considered orally bioavailable according to Lipinski’s rule of five. Compound 6 could be used as a lead for the synthesis of more effective hybrids.
1. Introduction
Inflammation is one of the body’s first lines of defense against harmful and foreign stimuli [1]. Dysregulation of the magnitude or duration of inflammation has been linked with the pathophysiology of various multifactorial conditions [2]. Several inflammatory mediators of the arachidonic acid cascade, particularly those of cyclooxygenase (COX) and lipoxygenase (LOX) pathways, have been associated with the pathogenesis and progression of many chronic inflammatory diseases [3,4]. Traditional non-steroidal anti-inflammatory drugs (tNSAIDs) were found to cause gastrointestinal bleeding due to COX-1 inhibition. A class of COX-2 selective inhibitors, known as Coxibs, was introduced in order to reduce the risk of gastrointestinal toxicity. Nevertheless, these agents had an increased risk of cardiovascular side effects owing to reduction in endothelial prostaglandin I2 (PGI2) and increased levels of platelet aggregator thromboxane A2 (TXA2) [4,5]. It has been indicated that inhibition of the cyclooxygenase pathway could divert arachidonate’s metabolism towards the lipoxygenase pathway, and vice versa, resulting in undesirable adverse effects [4].
Arguably, there is an urgent need for new anti-inflammatory agents with better safety profiles. In recent years there has been growing interest amongst the scientific community in pleiotropic approaches leading to drugs acting on multiple targets concurrently. The “one drug, multiple targets” philosophy could assist in developing novel compounds with better therapeutic profile against complex disease systems [6,7].
Cinnamic acid and its derivatives have been considered attractive potential multi-target agents by many research groups due to the multifunctional activities they present. Several medicinal applications of cinnamic-related molecules have appeared in the literature [8,9].
Pyrrole derivatives comprise an important class of heterocyclic compounds with a broad range of pharmaceutical applications [10], including antioxidant [11] and anti-inflammatory [12,13,14,15] activities. Two examples are the commercially available anti-inflammatory drugs tolmetin (Tolectin®) and ketorolac (Toradol®, Ketolac®), as shown in Figure 1.
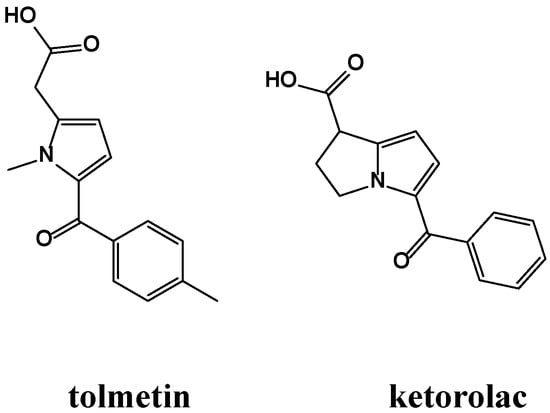
Figure 1.
NSAIDs containing the pyrrole moiety.
Chalcones have been reported to possess varied pharmacological activities, among them antioxidant [16,17] and anti-inflammatory [18,19]. These α,β-unsaturated carbonyl compounds have extensively been used as the building blocks of various heterocyclic compounds in the literature as well as for the synthesis of pyrroles.
It is commonly known that chronic inflammation and oxidative stress are inextricably interrelated. Overproduction of free radicals and depletion of the cellular antioxidant capacity can have detrimental effects on biomacromolecules, by means of lipid peroxidation, protein impairment and DNA mutation and damage [20]. Hence, it is imperative that research should focus on the development of new multifunctional agents combining antioxidant and anti-inflammatory activities.
In light of the above, we report the synthesis of a new pyrrole-cinnamic acid hybrid combining two pharmacophore units in a single molecule. The pyrrole moiety (4) was chosen for its potent inhibitory activity against COX-1 [15], while trans-cinnamic acid is well known for its pleiotropic activities [21,22]. Although the hybrid’s theoretically calculated lipophilicity (logP) value was found to be more than 5, it complies with the Lipinski’s rule of five (RO5) guidelines for oral bioavailability. The new compound (6) was evaluated for its antioxidant activity and its ability to inhibit soybean lipoxygenase. The findings suggest that the proposed hybrid could be used as a lead compound for the design and synthesis of more potent multifunctional agents.
2. Results and Discussion
2.1. Chemistry
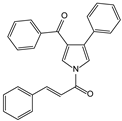
The synthesis of (E)-1-(3-benzoyl-4-phenyl-1H-pyrrol-1-yl)-3-phenylprop-2-en-1-one (6) is depicted on Scheme 1.
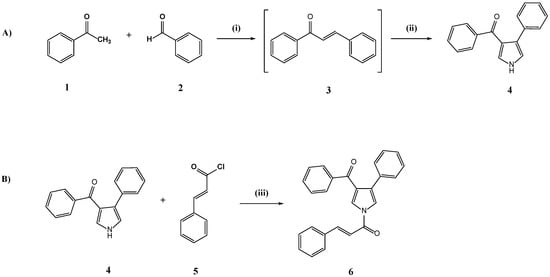
Scheme 1.
(A) One-pot synthesis of phenyl(4-phenyl-1H-pyrrol-3-yl)methanone (4). (i) LiOH.H2O, EtOH absolute, rt, 6 h. (ii) LiOH.H2O, p-toluenesulfonylmethyl isocyanide (TosMIC), rt, 17 h, yield 43% (B) Synthesis of (E)-1-(3-benzoyl-4-phenyl-1H-pyrrol-1-yl)-3-phenylprop-2-en-1-one (6). (iii) Et3N, 4-(dimethylamino)-pyridine (DMAP), dry CH2Cl2, Ar, rt, 24 h, yield 51%.
Starting from the commercially available acetophenone (1) and benzaldehyde (2), phenyl(4-phenyl-1H-pyrrol-3-yl)methanone (4) was obtained via a slightly modified one-pot reaction previously reported by Sharma et al. [23]. The first step involved an aldol condensation between the acetophenone enol and the electrophilic center of benzaldehyde in absolute EtOH, leading to the formation of the chalcone (3) intermediate. p-toluenesulfonylmethyl isocyanide (TosMIC) was added under basic conditions, generating the TosMIC anion which in turn reacted with the α,β-unsaturated carbonyl compound (3) providing the pyrrole (4) in 43% yield. In this procedure, a further 0.1 mmol of lithium hydroxide monohydrate (LiOH.H2O) was added, while the precipitate was purified by silica gel flash column chromatography (n-hexane-EtOAc, 4:1). The isolated phenyl(4-phenyl-1H-pyrrol-3-yl)methanone (4) was deprotonated under basic conditions in dry CH2Cl2 and subsequently reacted with cinnamoyl chloride (5), affording the desired cinnamic-pyrrole hybrid (6) in 43% yield as E-isomer [24].
The E-isomerism structure of (E)-1-(3-benzoyl-4-phenyl-1H-pyrrol-1-yl)-3-phenylprop-2-en-1-one (6) was confirmed by 1H, 13C-NMR and high-resolution mass-spectrometry (HRMS). Cinnamic moiety’s double-bond protons appear as two doublets at 8.08 and 7.16 ppm, with a J-coupling value at 15.4 Hz, indicative of the trans-isomerism. The aromatic signals at 7.89–7.86, 7.67–7.64, 7.62–7.61, 7.55–7.52, 7.47–7.45, 7.43, 7.42–7.39 and 7.32–7.28 correspond to the protons of the pyrrole ring and the three phenyl groups. In the 13C-NMR spectra, the signals of the benzoyl C=O and amide C=O are observed at 191.4 and 162.8 ppm, respectively. The remaining signals correspond to the double bond and aromatic carbons. In the HRMS spectrum, the found peaks values correspond to [M + H]⁺ (m/z = 378.1496) and [M + Na]⁺ (m/z = 400.1311) are consistent with the calculated ones ([M + H]⁺ 378.1489 and [M + Na]⁺ 400.1308), whereas elemental analysis supports the hybrid’s purity.
2.2. Physicochemical Studies
It has been demonstrated that theoretical determination of drug-likeness reduces drastically the ADMET (absorbance-distribution-elimination-metabolism-toxicity) related failures in the clinical trials [25]. Lipinski’s rule of five is a rule of thumb that helps to approach the drug-likeness of a compound with certain biological activity [26]. Thus, we found it interesting to theoretically calculate the molecular properties (https://www.molinspiration.com/cgi-bin/properties accessed date: 10 December 2021) of compound (6) (Table 1).

Table 1.
Drug-likeness of the synthesized hybrid 6. Molecular properties prediction—Lipinski “rule of five”.
Hybrid 6 presents one violation of Lipinski’s rules (logP > 5) and subsequently could be described as drug-like. The high lipophilicity often contributes to low solubility and poor cell membrane permeability and oral absorption. Additionally, the number of rotatable bonds is less than 10, suggesting that the molecule’s flexibility will not hinder its absorption and distribution. The topological polar surface area (TPSA), is highly correlated with the hydrogen bonding of a compound and has been applied for the prediction of intestinal absorption and blood–brain barrier penetration [28]. The TPSA of hybrid (6) is below the limits of 160 Å2 and 90 Å2, indicating good oral bioavailability and blood–brain barrier permeability, respectively.
Nonetheless, the aforementioned rules may not be applicable if a biological transporter is involved in the uptake of the drug or when the molecule is designed to inhibit molecular targets whose physiological substrate is quite lipophilic in nature, as in the case of lipoxygenase inhibitors.
The logarithm of brain-to-plasma concentration ratio (logBB) value of −0.29 was calculated from Clark’s modified Equation (1) [27].
where ClogP is the calculated octanol-water partitioning coefficient (miLogP) and TPSA is the topological polar surface area derived from https://www.molinspiration.com/cgi-bin/properties (accessed on 10 December 2021).
logBB = 0.152ClogP − 0.0148TPSA + 0.139
Hybrid 6 with logBB < −1.0 is poorly distributed through the blood–brain barrier.
2.3. Biological Evaluation
In the present study, the new cinnamic-pyrrole hybrid 6, as well as the parent molecule 4 were assessed with regard to their antioxidant activity and their ability to inhibit soybean lipoxygenase in comparison to the well-known reference compounds nordihydroguaiaretic acid (NDGA) and Trolox. For the sake of comparison, cinnamic acid’s (I) biological data are also included [29].
In vitro studies of lipid peroxidation are often conducted using azo compounds known to produce free radicals through spontaneous thermal decomposition. The water soluble 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) can decompose at 37 °C to generate an alkyl radical which in turn in the presence of oxygen will be converted to alkylperoxy radical that can cause lipid peroxidation [30]. The synthesized pyrrole 4 and hybrid 6 showed moderate radical-scavenging activity at 100 μM concentration (44% and 58%, respectively). The slight increase in the antioxidant activity of compound 6 could be attributed to the presence of the cinnamic moiety (Table 2).

Table 2.
% Inhibition of lipid peroxidation (ILP%) and in vitro inhibition of soybean lipoxygenase (IC50 μM or LOX Inh.%).
Due to lack of sufficiently purified mammalian lipoxygenases, in vitro inhibitory activities were measured against soybean lipoxygenase. In the current study, linoleic acid was used as a substrate. Soybean lipoxygenase-1 exhibits maximal activity at pH 9.0 and converts the substrate preferentially into the 13-hydroperoxide derivative. The formation of the conjugated hydroperoxy-diene product is detected by its absorbance at 234 nm. This spectrophotometric protocol works best at high pH values, where linoleic acid exists as a more soluble anionic salt. The pyrrole 4 showed moderate anti-LOX activity (100 μM), whereas the hybrid 6 presented good inhibitory activity (38 μM). This result supports the importance of the presence of the cinnamic group in the hybrid for the anti-LOX activity and the use of this hybrid as a lead compound.
3. Materials and Methods
3.1. General Information
All chemicals, solvents, chemical and biochemical reagents were of analytical grade and purchased from commercial suppliers (Merck, Merck KGaA, Darmstadt, Germany, Fluka, Sigma-Aldrich Laborchemikalien GmbH, Hannover, Germany, Alfa Aesar, Karlsruhe, Germany and Sigma-Aldrich, St. Louis, MO, USA). All starting materials were obtained from commercial sources (Fluka, Sigma-Aldrich Laborchemikalien GmbH, Merck) and used without further purification. Soybean lipoxygenase, sodium linoleate, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) were obtained from Sigma Chemical, Co. (St. Louis, MO, USA).
Melting points (uncorrected) were determined on a MEL-Temp II (Lab. Devices, Holliston, MA, USA). The in vitro tests were performed on a Perkin-Elmer Lamda 20 double beam spectrophotometer (Perkin-Elmer Corporation Ltd., Lane Beaconsfield, Bucks, UK). The proton nuclear magnetic resonance (1H-NMR) spectra were recorded at 500 MHz on an Agilent 500/54 spectrometer, Germany in CDCl3 or DMSO. The carbon nuclear magnetic resonance (13C-NMR) spectra were acquired at 126 MHz (Bruker Avance 500 spectrometer) in CDCl3 or DMSO with tetramethylsilane as an internal standard unless otherwise stated. Chemical shifts are expressed in δ (ppm) and coupling constants J in Hz. High resolution mass spectra (HRMS) were determined on an Agilent Q-TOF mass spectrometer, G6540B model with Dual AJS ESI-MS. Elemental analyses for C, H, and N gave values acceptably close to the theoretical values (±0.4%) in a Perkin-Elmer 240B CHN analyzer (Perkin-Elmer Corporation Ltd., Lane Beaconsfield, Bucks, UK).
Reactions were monitored by thin layer chromatography on 5554 F254 Silica gel/TLC cards (Merck and Fluka Chemie GmbH Buchs, Steinheim, Switzerland). For preparative thin layer chromatography (PTLC) silica gel 60 F254, plates 2 mm, Merck KGaAICH078057 were used.
3.2. Chemistry General Procedure
3.2.1. One-Pot Synthesis of Phenyl(4-phenyl-1H-pyrrol-3-yl)methanone (4)
The synthesis was performed according to reference [23]. Yield 105 mg (43%); white solid; Rf = 0.75 (n-hexane-ethyl acetate, 1:1, v/v); decomposes at 233–236 °C; 1H-NMR (500 MHz, DMSO-d6) (Figure S1) δ 11.63 (brs, 1H), 7.73 (d, J = 7.5 Hz, 2H), 7.58–7.53 (m, 1H), 7.48–7.43 (m, 2H), 7.38–7.35 (m, 2H), 7.27–7.21 (m, 3H), 7.18–7.14 (m, 1H), 7.09–7.07 (m, 1H); 13C-NMR (126 MHz, DMSO-d6) (Figure S2) δ 190.3, 139.9, 135.2, 131.5, 128.9, 128.34, 128.1, 128.1, 127.7, 125.6, 125.5, 120.5, 119.6.
3.2.2. Synthesis of (E)-1-(3-Benzoyl-4-phenyl-1H-pyrrol-1-yl)-3-phenylprop-2-en-1-one (6)
Triethylamine (0.02 mL, 0.14 mmol) and 4-(dimethylamino)-pyridine (1.73 mg, 0.014 mmol) were added to a solution of phenyl(4-phenyl-1H-pyrrol-3-yl)methanone (39.76 mg, 0.16 mmol) in dry CH2Cl2 (4 mL) [24]. The mixture was left stirring at room temperature for 10 min and subsequently cinnamoyl chloride (20.24 mg, 0.12 mmol) was added dropwise under argon atmosphere. The reaction mixture was allowed to stir at room temperature for 24 h. Upon completion, the mixture was dissolved in Et2O (30 mL) and washed with NaHSO4 10% (3 × 25 mL), NaHCO3 10% (3 × 25 mL) solutions, water (2 × 25 mL) and brine (3 × 25 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was treated with warm ethyl acetate and n-hexane. The precipitate was filtered out, washed with water and, consequently, purified by preparative thin layer chromatography (n-hexane-ethyl acetate, 4:1, v/v) and recrystallized by a mixture of ethyl acetate and n-hexane to give pure hybrid (6). Yield 23.35 mg (51%); amorphous orange solid; Rf = 0.65 (n-hexane-ethyl acetate, 6:1, v/v); 1H-NMR (500 MHz, CDCl3) (Figure S3) δ 8.08 (d, J = 15.4 Hz, 1H), 7.89–7.86 (m, 3H), 7.67–7.64 (m, 2H), 7.62–7.61 (m, 1H), 7.55–7.52 (m, 1H), 7.47–7.45 (m, 2H), 7.43 (s, 1H), 7.42–7.39 (m, 4H), 7.32–7.28 (m, 3H), 7.16 (d, J = 15.4 Hz, 1H); 13C-NMR (126 MHz, CDCl3) (Figure S4) δ 191.4, 162.8, 149.5, 138.7, 134.0, 133.2, 132.7, 131.7, 130.3, 129.8, 129.3, 128.9, 128.6, 128.5, 128.4, 127.4, 126.2, 125.7, 118.5, 114.5. Elemental analysis Calculated: C 82.74, H 5.07, N 3.71. Found C 82.71, H 5.17, N 3.78. HRMS (Q-TOF-ESI) (Figure S5) m/z: [M + Na]+ Calculated for C26H19NO2: 400.1308, found 400.1311; m/z: [M + H]+ Calculated for C26H19NO2: 378.1489, found 378.1496.
3.3. Biological In Vitro Assays
For the in vitro biological evaluation, a stock solution (10 mM, 1% DMSO in the appropriate buffer with the tested compound diluted under sonication) was prepared, from which several dilutions were made with the appropriate buffer.
Each experiment was performed at least twice and the standard deviation of absorbance did not exceed 10%.
3.3.1. Inhibition of Linoleic Acid Lipid Peroxidation
2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH) was used as a controllable source of thermally produced alkylperoxy free radicals by oxidation of sodium linoleate in an aqueous solution. The rate of oxidation at 37 °C was monitored by recording the increase in absorption at 234 nm caused by the formation of conjugated diene hydroperoxides [29,30]. The results were compared to the reference compound, Trolox (93%) (Table 2).
3.3.2. Inhibition of Soybean Lipoxygenase In Vitro
The tested compounds were dissolved in DMSO (100 μΜ) and incubated at room temperature with sodium linoleate (0.1 mL) as a substrate and 0.2 mL of a soybean lipoxygenase solution in a buffer solution of Tris:HCl (pH 9.00). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard inhibitor NDGA (IC50 = 0.45 μM) [31]. The results are given in Table 2.
4. Conclusions
In the present study, a new cinnamic-pyrrole hybrid was synthesised as a potential antioxidant agent with LOX inhibitory activity. Its chemical structure was verified by NMR and mass spectra. The in vitro biological experiments showed moderate anti-lipid peroxidation activity (58%) in combination with good anti-LOX activity (38 µM). These findings compared to the moderate biological activities of the pyrrole precursor, indicate the biological importance of the combination of a pyrrolyl ring with a cinnamic moiety in a hybrid molecule and the use of this hybrid as a lead compound. Further investigations are in progress to determine the anti-inflammatory activity of the hybrid.
Supplementary Materials
The following supporting information can be downloaded. Figure S1: 1H-NMR (500 MHz, DMSO-d6) spectrum of compound 4; Figure S2: 13C-NMR (126 MHz, DMSO-d6) spectrum of compound 4; Figure S3: 1H-NMR (500 MHz, CDCl3) spectrum of compound 6; Figure S4: 13C-NMR (126 MHz, CDCl3) spectrum of compound 6; Figure S5: HRMS spectrum of compound 6.
Author Contributions
D.H.-L. contributed to the supervision, design, conceptualization, project administration, validation and writing. V.N. contributed to the writing, synthesis, biological evaluation and analysis of the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors are grateful to Catherine Gabriel (Center for Research of the Structure of Matter, Magnetic Resonance Laboratory, Department of Chemical Engineering, Aristotle University of Thessaloniki) for recording the HRMS spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonald, D.R.; Levy, O. 3-Innate Immunity. In Clinical Immunology, 5th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier: London, UK, 2019; pp. 39–53.e1. ISBN 978-0-7020-6896-6. [Google Scholar]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.-B.; Gualde, N. Arachidonic-Acid-Derived Eicosanoids: Roles in Biology and Immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef]
- P, J.J.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer Anti-Inflammatory Therapy through Dual COX-2/5-LOX Inhibitors: A Structure-Based Approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Marsico, F.; Paolillo, S.; Filardi, P.P. NSAIDs and Cardiovascular Risk. J. Cardiovasc. Med. 2017, 18, e40. [Google Scholar] [CrossRef]
- Reddy, A.S.; Zhang, S. Polypharmacology: Drug Discovery for the Future. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-Target Therapeutics: When the Whole Is Greater than the Sum of the Parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef]
- Pontiki, E.; Peperidou, A.; Fotopoulos, I.; Hadjipavlou-Litina, D. Cinnamate Hybrids: A Unique Family of Compounds with Multiple Biological Activities. Curr. Pharm. Biotechnol. 2018, 19, 1019–1048. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Gholap, S.S. Pyrrole: An Emerging Scaffold for Construction of Valuable Therapeutic Agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
- Mallikarjuna Reddy, G.; Camilo, A.; Raul Garcia, J. Pyrrole-2,5-Dione Analogs as a Promising Antioxidant Agents: Microwave-Assisted Synthesis, Bio-Evaluation, SAR Analysis and DFT Studies/Interpretation. Bioorg. Chem. 2021, 106, 104465. [Google Scholar] [CrossRef]
- Sarg, M.T.; Koraa, M.M.; Bayoumi, A.H.; Gilil, S.M.A.E. Synthesis of Pyrroles and Condensed Pyrroles as Anti-Inflammatory Agents with Multiple Activities and Their Molecular Docking Study. Open J. Med. Chem. 2015, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-T.; Mou, X.-Q.; Xi, Q.-M.; Liu, W.-T.; Liu, W.-F.; Sheng, Z.-J.; Zheng, X.; Zhang, K.; Du, Z.-Y.; Zhao, S.-Q.; et al. Anti-Inflammatory Activity Effect of 2-Substituted-1,4,5,6-Tetrahydrocyclopenta [b] Pyrrole on TPA-Induced Skin Inflammation in Mice. Bioorg. Med. Chem. Lett. 2016, 26, 5334–5339. [Google Scholar] [CrossRef]
- Konstantinidou, M.; Gkermani, A.; Hadjipavlou-Litina, D. Synthesis and Pharmacochemistry of New Pleiotropic Pyrrolyl Derivatives. Molecules 2015, 20, 16354–16374. [Google Scholar] [CrossRef]
- Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. The Pyrrole Moiety as a Template for COX-1/COX-2 Inhibitors. Eur. J. Med. Chem. 2000, 35, 499–510. [Google Scholar] [CrossRef]
- Polo, E.; Ibarra-Arellano, N.; Prent-Peñaloza, L.; Morales-Bayuelo, A.; Henao, J.; Galdámez, A.; Gutiérrez, M. Ultrasound-Assisted Synthesis of Novel Chalcone, Heterochalcone and Bis-Chalcone Derivatives and the Evaluation of Their Antioxidant Properties and as Acetylcholinesterase Inhibitors. Bioorg. Chem. 2019, 90, 103034. [Google Scholar] [CrossRef]
- Kumar, C.S.C.; Loh, W.-S.; Ooi, C.W.; Quah, C.K.; Fun, H.-K. Structural Correlation of Some Heterocyclic Chalcone Analogues and Evaluation of Their Antioxidant Potential. Molecules 2013, 18, 11996–12011. [Google Scholar] [CrossRef] [Green Version]
- Rashid, H.U.; Xu, Y.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising Anti-Inflammatory Effects of Chalcones via Inhibition of Cyclooxygenase, Prostaglandin E2, Inducible NO Synthase and Nuclear Factor Κb Activities. Bioorg. Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef]
- Rücker, H.; Al-Rifai, N.; Rascle, A.; Gottfried, E.; Brodziak-Jarosz, L.; Gerhäuser, C.; Dick, T.P.; Amslinger, S. Enhancing the Anti-Inflammatory Activity of Chalcones by Tuning the Michael Acceptor Site. Org. Biomol. Chem. 2015, 13, 3040–3047. [Google Scholar] [CrossRef] [Green Version]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated Relationship of Inflammation and Oxidative Stress in Major Depression. Brain. Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Shang, B.; Li, Y.; Zhen, Y. Inhibition of Histone Deacetylases by Trans-Cinnamic Acid and Its Antitumor Effect against Colon Cancer Xenografts in Athymic Mice. Mol. Med. Rep. 2016, 13, 4159–4166. [Google Scholar] [CrossRef] [Green Version]
- Anlar, H.G.; Bacanli, M.; Çal, T.; Aydin, S.; Ari, N.; Bucurgat, Ü.Ü.; Başaran, A.A.; Başaran, A.N. Effects of Cinnamic Acid on Complications of Diabetes. Turk. J. Med. Sci. 2018, 48, 168–177. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, K.; Chouhan, M.; Grover, V.; Nair, V.A. Lithium Hydroxide Mediated Synthesis of 3,4-Disubstituted Pyrroles. RSC Adv. 2013, 3, 14521–14527. [Google Scholar] [CrossRef]
- D’Silva, C.; Iqbal, R. A New Method to N-Arylmethylenepyrroles from N-Acylpyrroles. Synthesis 1996, 1996, 457–458. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Clark, D.E. Rapid Calculation of Polar Molecular Surface Area and Its Application to the Prediction of Transport Phenomena. 2. Prediction of Blood-Brain Barrier Penetration. J. Pharm. Sci. 1999, 88, 815–821. [Google Scholar] [CrossRef]
- Fernandes, J.; Gattass, C.R. Topological Polar Surface Area Defines Substrate Transport by Multidrug Resistance Associated Protein 1 (MRP1/ABCC1). J. Med. Chem. 2009, 52, 1214–1218. [Google Scholar] [CrossRef]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget Molecular Hybrids of Cinnamic Acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef]
- Liégeois, C.; Lermusieau, G.; Collin, S. Measuring Antioxidant Efficiency of Wort, Malt, and Hops against the 2,2′-Azobis (2-Amidinopropane) Dihydrochloride-Induced Oxidation of an Aqueous Dispersion of Linoleic Acid. J. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).