2-((E)-2-((E)-4-Chloro-5-(2-((E)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene) ethylidene)-1,1-dimethyl-1,2,5,6-tetrahydropyridin-1-ium-3-yl)vinyl)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
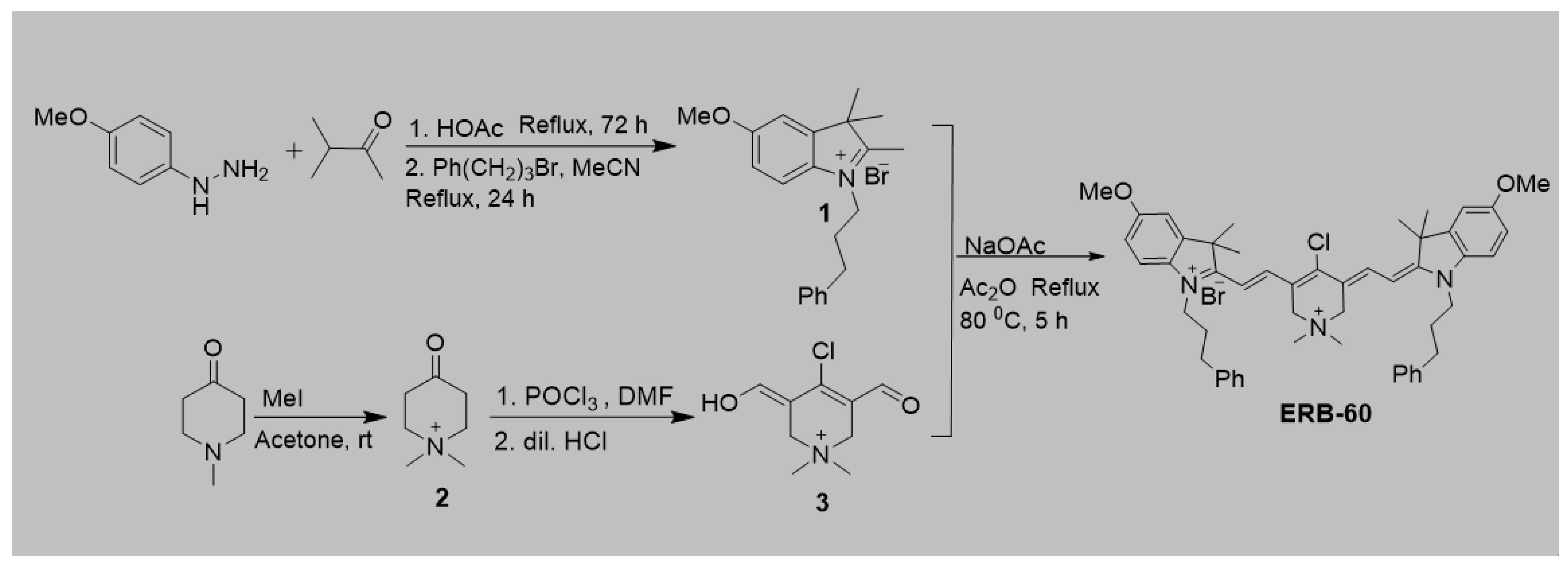
2.2. Physicochemical and Optical Properties of ERB-60
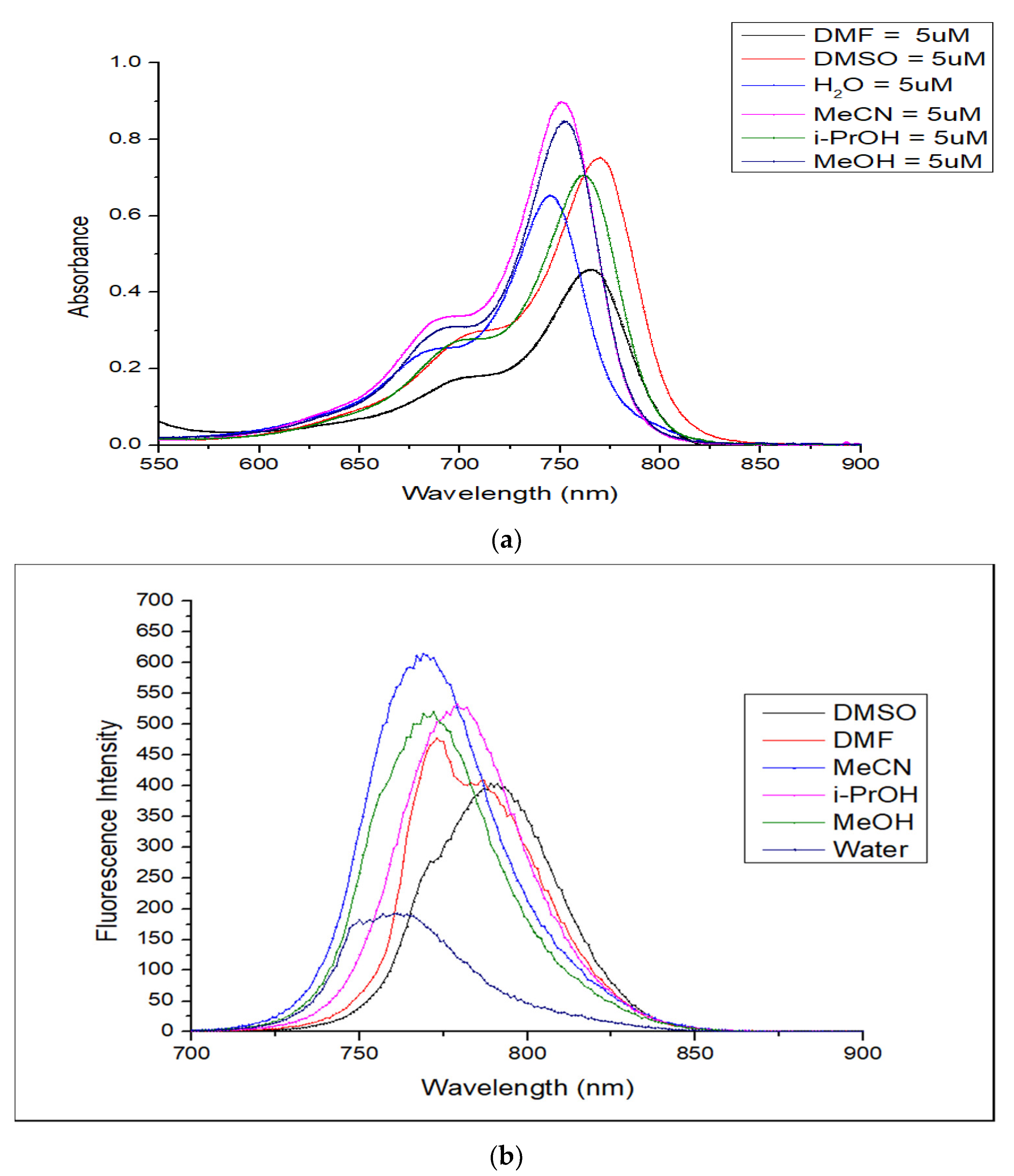
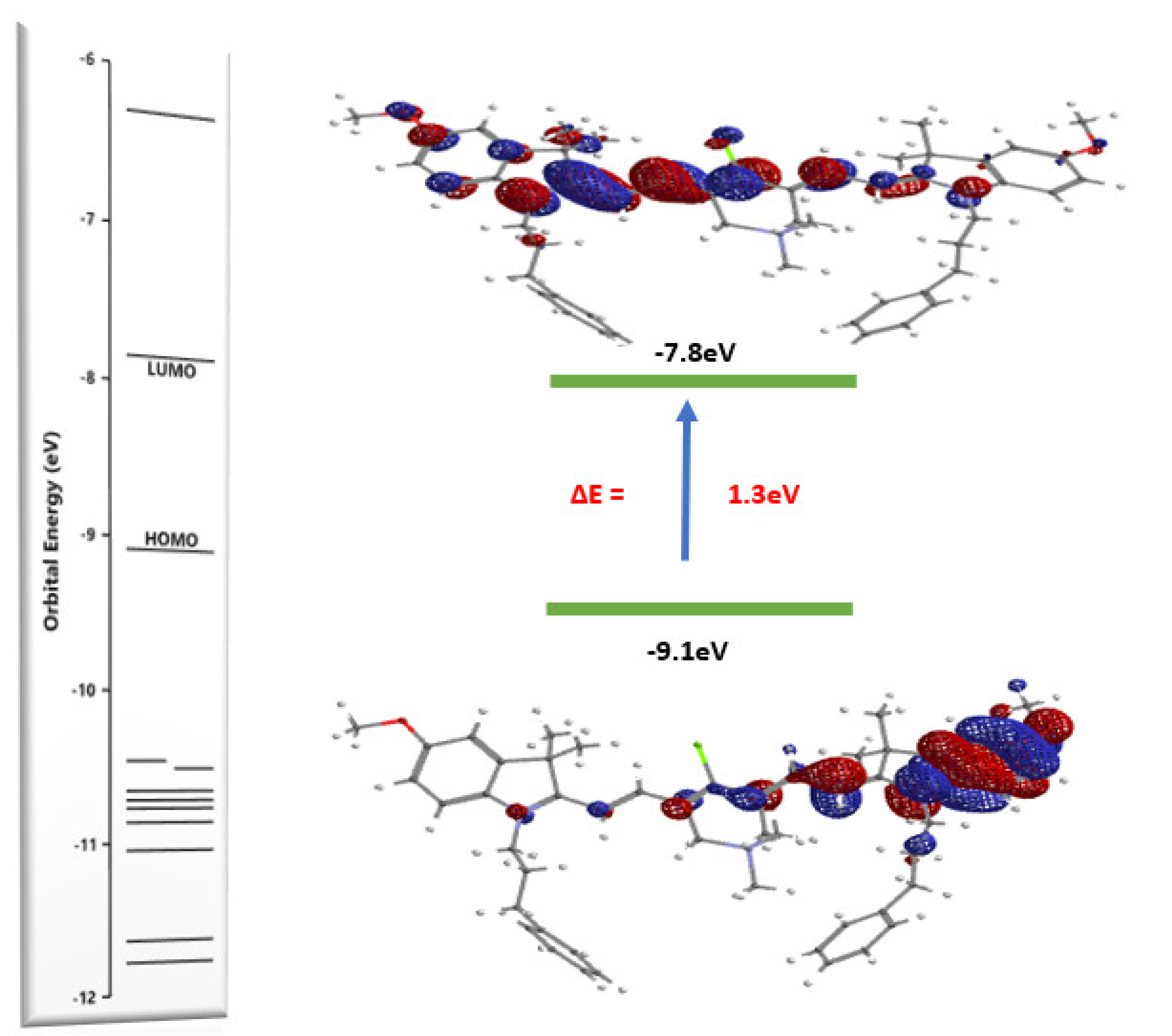
2.3. Optical Properties
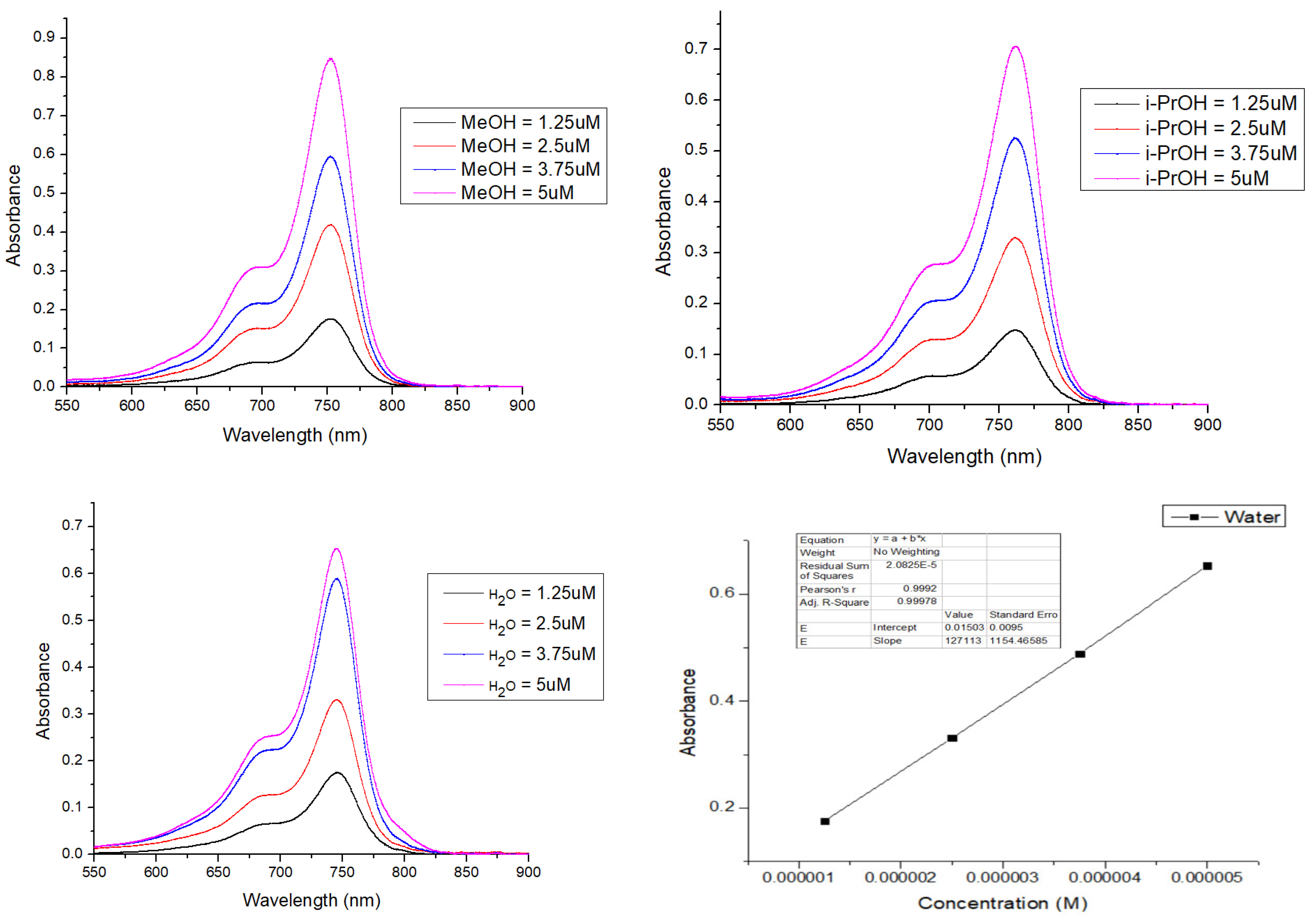
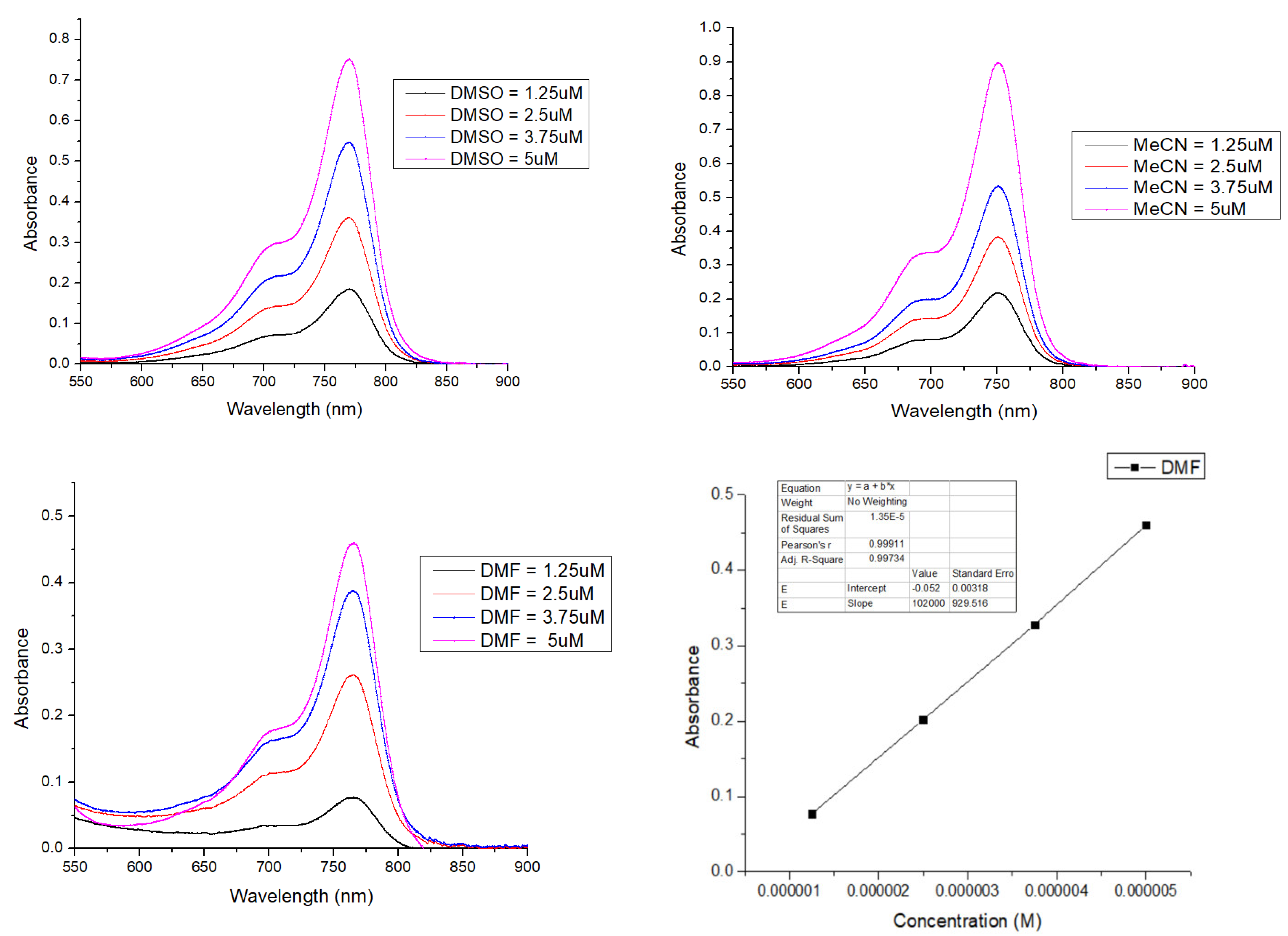
2.4. Solvatochromism Properties of ERB-60
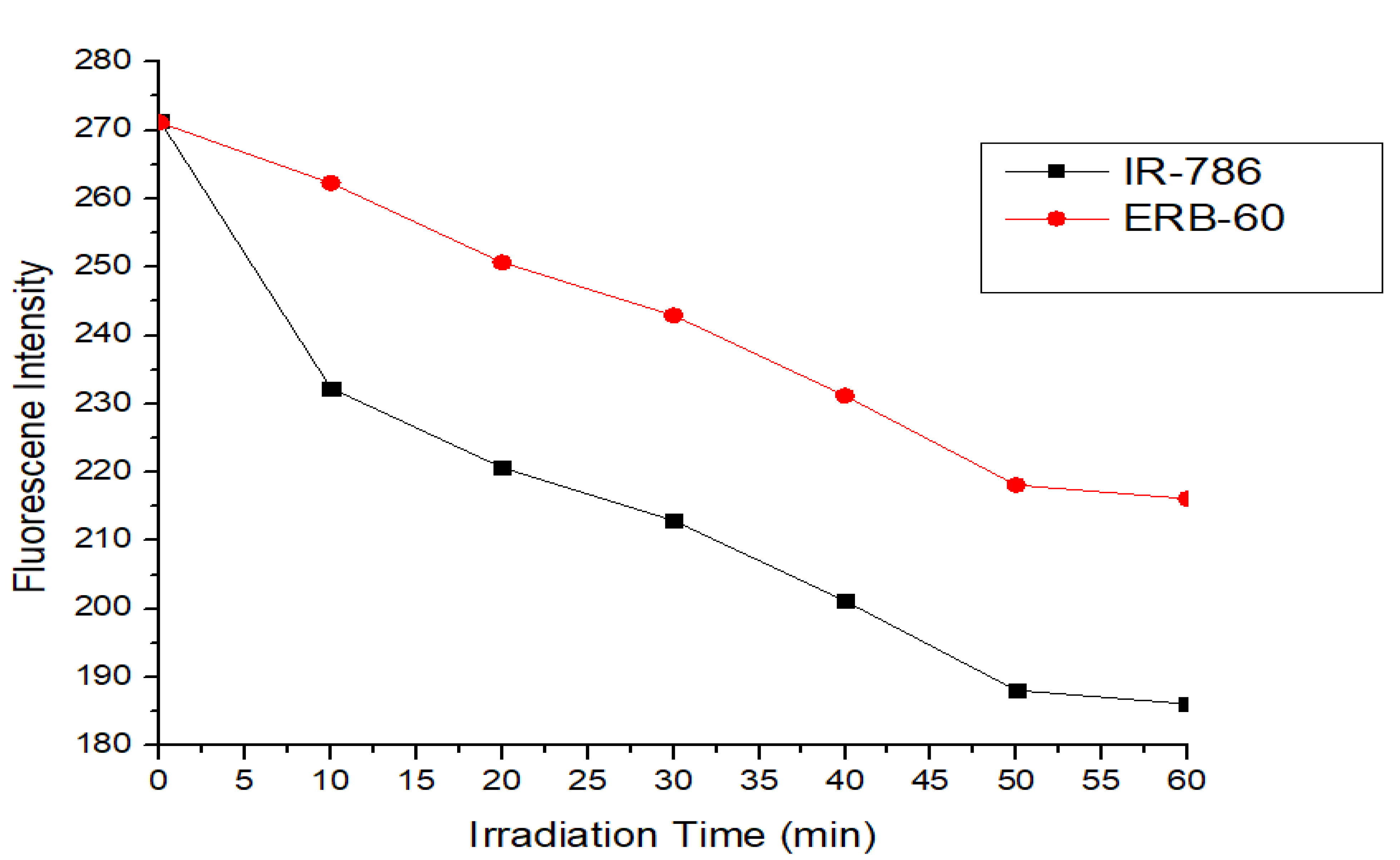
3. Photostability of ERB-60
4. Materials and Methods
4.1. Preparation of Stock Solution
4.2. Determination of Molar Absorptivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahoulou, E.O.; Drinkard, K.K.; Basnet, K.; St Lorenz, A.; Taratula, O.; Henary, M.; Grant, K.B. DNA Photocleavage in the Near-Infrared Wavelength Range by 2-Quinolinium Dicarbocyanine Dyes. Molecules 2020, 25, 2926. [Google Scholar] [CrossRef]
- Deligeorgiev, T.G.; Vasilev, A.; Drexhage, K.H. Synthesis of novel cyanine dyes containing carbamoylethyl component—Noncovalent labels for nucleic acids detection. Dyes Pigm. 2007, 74, 320–328. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Owens, E.A.; Wada, H.; Henary, M.; Handgraaf, H.J.; Vahrmeijer, A.L.; Frangioni, J.V.; Choi, H.S. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med. 2015, 21, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoon, H.; Eric, A.O.; Hideyuki, W.; Andrew, L.; Gwang, P.; Min Ho, P.; John, V.F.; Maged, H.; Hak, S.C. Cartilage-Specific Near-Infrared Fluorophores for Biomedical Imaging. Angew. Chem. Int. Ed. Engl. 2015, 54, 8648–8652. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S.; et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153. [Google Scholar] [CrossRef]
- Hoon, H.; Hideyuki, W.; Kai, B.; Julien, G.; Yogesh, Y.; Matt, L.; Maged, H.; John, V.F.; Hak, S.C. Phosphonated Near-Infrared Fluorophores for Biomedical Imaging of Bone. Angew. Chem. 2014, 126, 10844–10848. [Google Scholar] [CrossRef] [Green Version]
- Laramie, M.D.; Fouts, B.L.; MacCuaig, W.M.; Buabeng, R.E.; Jones, M.A.; Mukherjee, P.; Behkam, B.; McNally, L.R.; Henary, M. Improved pentamethine cyanine nanosensors for optoacoustic imaging of pancreatic cancer. Sci. Rep. 2021, 11, 4366. [Google Scholar] [CrossRef]
- Buabeng, R.E.; Maged, H. Developments of small molecules as inhibitors for carbonic anhydrase isoforms. Bioorg. Med. Chem. 2021, 39, 116140. [Google Scholar] [CrossRef]
- Levitz, A.; Marmarchi, F.; Henary, M. Introduction of various substitutions to the methine bridge of heptamethine cyanine dyes Via substituted dianil linkers. Photochem. Photobiol. Sci. 2018, 17, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Hyun, H.; Tawney, J.G.; Choi, H.S.; Henary, M. Correlating molecular character of NIR imaging agents with tissue-specific uptake. J. Med. Chem. 2015, 58, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-specific near-infrared fluorescence imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Park, G.K.; Lee, J.H.; Levitz, A.; El Fakhri, G.; Hwang, N.S.; Henary, M.; Choi, H.S. Lysosome-Targeted Bioprobes for Sequential Cell Tracking from Macroscopic to Microscopic Scales. Adv. Mater. 2019, 31, e1806216. [Google Scholar] [CrossRef]
- Andrew, L.; Cory, H.; Eduardo, S.; Maged, H. “Turn on” fluorescence response of monomethine cyanines caused by noncovalent binding to ct-DNA. Dyes Pigm. 2017, 145, 202–207. [Google Scholar]
- Yogesh, Y.; Andrew, L.; Sanam, D.; Ritu, A.; Maged, H. Effects of heterocyclic N-alkyl chain length on cancer cell uptake of near infrared heptamethine cyanine dyes. Dyes Pigm. 2017, 145, 307–314. [Google Scholar]
- Levitz, A.; Marmarchi, F.; Henary, M. Synthesis and optical properties of near-infrared meso-phenyl-substituted symmetric heptamethine cyanine dyes. Molecules 2018, 23, 226. [Google Scholar] [CrossRef] [Green Version]
- Kiryl, D.P.; Vladislav, V.V. Guide to red fluorescent proteins and biosensors for flow cytometry. Methods Cell. Biol. 2011, 102, 431–461. [Google Scholar]
- Haiying, Z.; George, T.; Dennis, S.; David, M.K.; Hairong, Z.; Tommy, D.; William, C.E.; Mikhail, Y.B. Cell-free measurements of brightness of fluorescently labeled antibodies. Sci. Rep. 2017, 7, 41819. [Google Scholar]
- Alvaro, S.a.n.c.h.e.z.-A.; Yan, C.; Joachim, D.M. Molecular brightness determined from a generalized form of Mandel’s Q-parameter. Biophys. J. 2005, 89, 3531–3547. [Google Scholar]
- Thavornpradit, S.; Usama, S.M.; Park, G.K.; Shrestha, J.P.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. QuatCy: A Heptamethine Cyanine Modification With Improved Characteristics. Theranostics 2019, 9, 2856–2867. [Google Scholar] [CrossRef]
- Henary, M.; Mojzych, M. Stability and reactivity of polymethine dyes in solution. In Heterocyclic Polymethine Dyes; Topics in Heterocyclic Chemistry; Strekowski, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 14, pp. 221–238. [Google Scholar]
- Shershov, V.E.; Spitsyn, M.A.; Kuznetsova, V.E.; Timofeev, E.N.; Ivashkina, O.A.; Abramov, I.S.; Nasedkina, T.V.; Zasedatelev, A.S.; Chudinov, A.V. Near-infrared heptamethine cyanine dyes. Synthesis, spectroscopic characterization, thermal properties and photostability. Dyes Pigm. 2013, 97, 353–360. [Google Scholar] [CrossRef]
- Xiaozhong, M.; Matthew, L.; Maged, H. Synthesis, optical properties and cytotoxicity of meso-heteroatom substituted IR-786 analogs. Bioorg. Med. Chem. Lett. 2018, 28, 509–514. [Google Scholar]
- Parul, R.; Yuriy, P.B.; Ryan, D.R.; Yangchun, L.; William, S.B.; Deepti, S.; Michael, G.S.; Igor, L.; Stephen, H.F. Selective imaging and killing of cancer cells with protein-activated near-infrared fluorescing nanoparticles. Macromol. Biosci. 2011, 11, 927–937. [Google Scholar]
- Gala, C.; Maged, H.; Gabor, P. The effect of varying short-chain alkyl substitution on the molar absorptivity and quantum yield of cyanine dyes. Anal. Chem. Insights 2011, 6, 29–36. [Google Scholar]
| ID | logD | Polarizability | Dipole Moment | n rot. | Molecular Volume | Molecular Surface Area | Molecular Weight |
|---|---|---|---|---|---|---|---|
| ERB-60 | 3.9 | 92.36 | 17.77 | 13 | 785.52 | 1214 | 862 |
| Solvents | λabs nm | λem nm | Extinction Coefficient ε (M−1 cm−1) | Stokes Shift (nm) | Quantum Yield (QY) | Molecular Brightness (M−1 cm−1) |
|---|---|---|---|---|---|---|
| H2O | 745 | 761 | 127,113 | 16 | 0.098 | 12,457 |
| MeOH | 752 | 769 | 172,240 | 17 | 0.249 | 42,888 |
| i-PrOH | 761 | 779 | 149,920 | 18 | 0.250 | 37,840 |
| MeCN | 751 | 772 | 179,920 | 21 | 0.310 | 55,775 |
| DMF | 766 | 773 | 102,000 | 7 | 0.268 | 27,336 |
| DMSO | 770 | 789 | 151,360 | 19 | 0.164 | 24,823 |
| ICG [23,24] | 783 | 802 | 122,000 | 19 | 0.036 | 4392 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buabeng, E.R.; Henary, M. 2-((E)-2-((E)-4-Chloro-5-(2-((E)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene) ethylidene)-1,1-dimethyl-1,2,5,6-tetrahydropyridin-1-ium-3-yl)vinyl)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium. Molbank 2021, 2021, M1270. https://doi.org/10.3390/M1270
Buabeng ER, Henary M. 2-((E)-2-((E)-4-Chloro-5-(2-((E)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene) ethylidene)-1,1-dimethyl-1,2,5,6-tetrahydropyridin-1-ium-3-yl)vinyl)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium. Molbank. 2021; 2021(3):M1270. https://doi.org/10.3390/M1270
Chicago/Turabian StyleBuabeng, Emmanuel Ramsey, and Maged Henary. 2021. "2-((E)-2-((E)-4-Chloro-5-(2-((E)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene) ethylidene)-1,1-dimethyl-1,2,5,6-tetrahydropyridin-1-ium-3-yl)vinyl)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium" Molbank 2021, no. 3: M1270. https://doi.org/10.3390/M1270
APA StyleBuabeng, E. R., & Henary, M. (2021). 2-((E)-2-((E)-4-Chloro-5-(2-((E)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)indolin-2-ylidene) ethylidene)-1,1-dimethyl-1,2,5,6-tetrahydropyridin-1-ium-3-yl)vinyl)-5-methoxy-3,3-dimethyl-1-(3-phenylpropyl)-3H-indol-1-ium. Molbank, 2021(3), M1270. https://doi.org/10.3390/M1270







