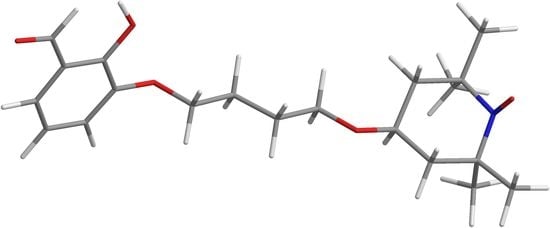
2-Hydroxy-3-(4-oxy(2,2,6,6-tetramethylpiperidin-1-oxyl)butoxy)benzaldehyde
Abstract
:1. Introduction
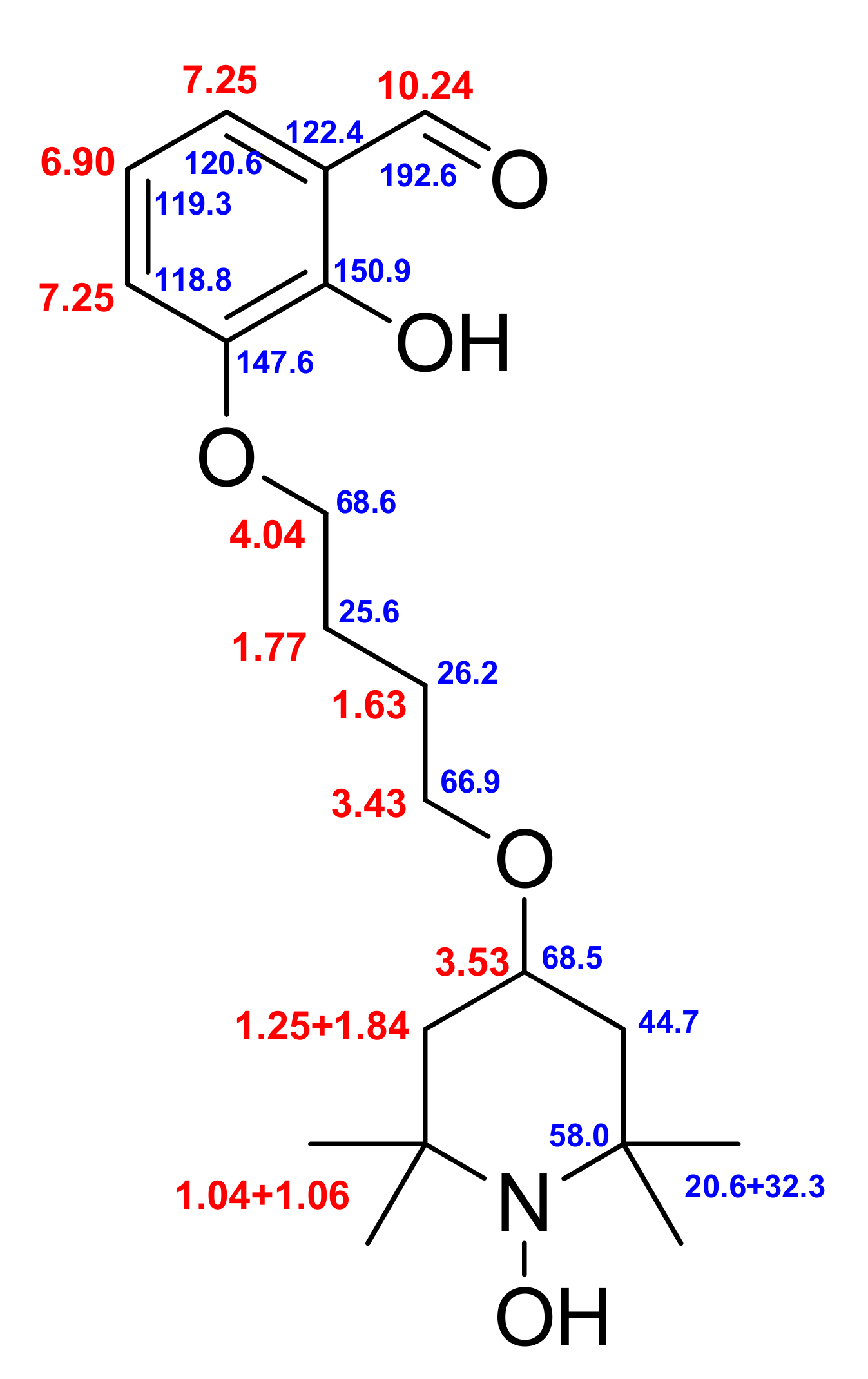
2. Results
3. Materials and Methods
3.1. General Consideration
3.2. Synthesis of 2-Hydroxy-3-(4-oxy(2,2,6,6-tetramethylpiperidin-1-oxyl)butoxy)benzaldehyde
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Zhu, Z.; Li, X.; Li, J. Electropolymerization and energy storage of poly[Ni(salphen)]/MWCNT composite materials for supercapacitors. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Alekseeva, E.V.; Chepurnaya, I.A.; Malev, V.V.; Timonov, A.M.; Levin, O.V. Polymeric nickel complexes with salen-type ligands for modification of supercapacitor electrodes: Impedance studies of charge transfer and storage properties. Electrochim. Acta 2017, 225, 378–391. [Google Scholar] [CrossRef]
- Yan, G.; Li, J.; Zhang, Y.; Gao, F.; Kang, F. Electrochemical polymerization and energy storage for Poly[Ni(salen)] as supercapacitor electrode material. J. Phys. Chem. C 2014, 118, 9911–9917. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Kang, F.; Zhang, Y.; Wang, X.; Ye, F.; Yang, J. Preparation and characterization of a Poly[Ni(salen)]/Multiwalled carbon nanotube composite by in situ electropolymerization as a capacitive material. J. Phys. Chem. C 2011, 115, 11822–11829. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Zhang, Y.; Kang, F.; Wang, X.; Ye, F.; Yang, J. Electropolymerization of Ni(salen) on carbon nanotube carrier as a capacitive material by pulse potentiostatic method. Sci. China Chem. 2012, 55, 1338–1344. [Google Scholar] [CrossRef]
- Tedim, J.; Gonçalves, F.; Pereira, M.F.R.; Figueiredo, J.L.; Moura, C.; Freire, C.; Hillman, A.R. Preparation and characterization of Poly[Ni(salen)(crown receptor)]/multi-walled carbon nanotube composite films. Electrochim. Acta 2008, 53, 6722–6731. [Google Scholar] [CrossRef]
- Vereschagin, A.A.; Sizov, V.V.; Vlasov, P.S.; Alekseeva, E.V.; Konev, A.S.; Levin, O.V. Water-stable [Ni(salen)]-type electrode material based on phenylazosubstituted salicylic aldehyde imine ligand. New J. Chem. 2017, 41, 13918–13928. [Google Scholar] [CrossRef] [Green Version]
- Vilas-Boas, M.; Santos, I.C.; Henderson, M.J.; Freire, C.; Hillman, A.R.; Vieil, E. Electrochemical behavior of a new precursor for the design of Poly[Ni(salen)]-based modified electrodes. Langmuir 2003, 19, 7460–7468. [Google Scholar] [CrossRef] [Green Version]
- Tedim, J.; Carneiro, A.; Bessada, R.; Patrício, S.; Magalhães, A.L.; Freire, C.; Gurman, S.J.; Hillman, A.R. Correlating structure and ion recognition properties of [Ni(salen)]-based polymer films. J. Electroanal. Chem. 2007, 610, 46–56. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Alekseeva, E.V.; Vereshchagin, A.A.; Volkov, A.I.; Vlasov, P.S.; Konev, A.S.; Levin, O.V. Nickel-salen type polymers as cathode materials for rechargeable lithium batteries. Macromol. Chem. Phys. 2017, 218, 1700361. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Vlasov, P.S.; Konev, A.S.; Yang, P.; Grechishnikova, G.A.; Levin, O.V. Novel highly conductive cathode material based on stable-radical organic framework and polymerized nickel complex for electrochemical energy storage devices. Electrochim. Acta 2019, 295, 1075–1084. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Lukyanov, D.A.; Kulikov, I.R.; Panjwani, N.A.; Alekseeva, E.A.; Behrends, J.; Levin, O.V. The fast and the capacious: A [Ni(Salen)]-TEMPO redox-conducting polymer for organic batteries. Batter. Supercaps 2021, 4, 336–346. [Google Scholar] [CrossRef]
- Li, F.; Gore, D.N.; Wang, S.; Lutkenhaus, J.L. Unusual internal electron transfer in conjugated radical polymers. Angew. Chem. 2017, 129, 9988–9991. [Google Scholar] [CrossRef]
- Karlsson, C.; Huang, H.; Strømme, M.; Gogoll, A.; Sjödin, M. Impact of linker in polypyrrole/quinone conducting redox polymers. RSC Adv. 2015, 5, 11309–11316. [Google Scholar] [CrossRef]
- Han, S.; Zhang, F.-F.; Qian, H.-Y.; Chen, L.-L.; Pu, J.-B.; Xie, X.; Chen, J.-Z. Design, syntheses, structure–activity relationships and docking studies of coumarin derivatives as novel selective ligands for the CB2 receptor. Eur. J. Med. Chem. 2015, 93, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, J.; Chen, H.; Feng, L.; Jing, R.; Lu, M.; Hu, B.; Ji, J. Magnetic superhydrophobic polymer nanosphere cage as a framework for miceller catalysis in biphasic media. ChemCatChem 2014, 6, 1626–1634. [Google Scholar] [CrossRef]
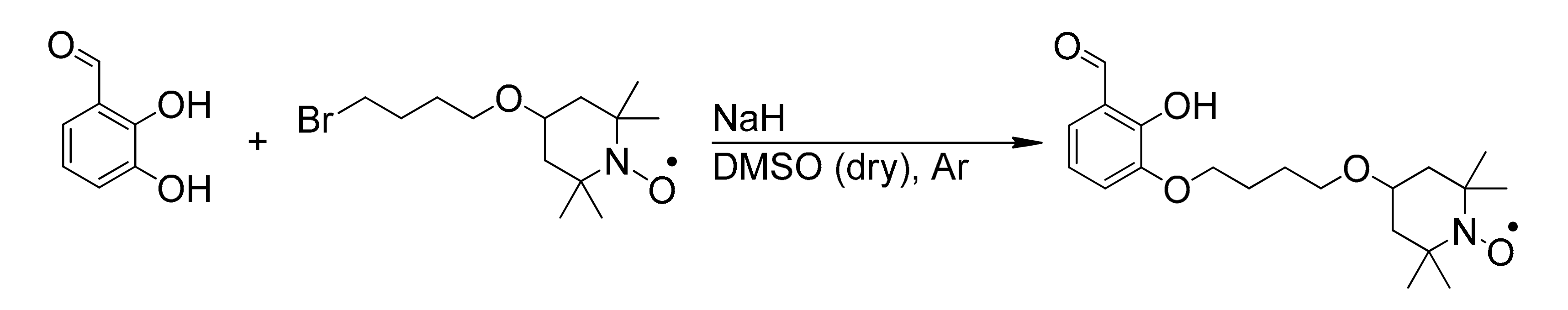
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, A.A.; Novoselova, J.V.; Kalnin, A.Y.; Lukyanov, D.A. 2-Hydroxy-3-(4-oxy(2,2,6,6-tetramethylpiperidin-1-oxyl)butoxy)benzaldehyde. Molbank 2021, 2021, M1245. https://doi.org/10.3390/M1245
Vereshchagin AA, Novoselova JV, Kalnin AY, Lukyanov DA. 2-Hydroxy-3-(4-oxy(2,2,6,6-tetramethylpiperidin-1-oxyl)butoxy)benzaldehyde. Molbank. 2021; 2021(3):M1245. https://doi.org/10.3390/M1245
Chicago/Turabian StyleVereshchagin, Anatoliy A., Julia V. Novoselova, Arseniy Y. Kalnin, and Daniil A. Lukyanov. 2021. "2-Hydroxy-3-(4-oxy(2,2,6,6-tetramethylpiperidin-1-oxyl)butoxy)benzaldehyde" Molbank 2021, no. 3: M1245. https://doi.org/10.3390/M1245
APA StyleVereshchagin, A. A., Novoselova, J. V., Kalnin, A. Y., & Lukyanov, D. A. (2021). 2-Hydroxy-3-(4-oxy(2,2,6,6-tetramethylpiperidin-1-oxyl)butoxy)benzaldehyde. Molbank, 2021(3), M1245. https://doi.org/10.3390/M1245