Abstract
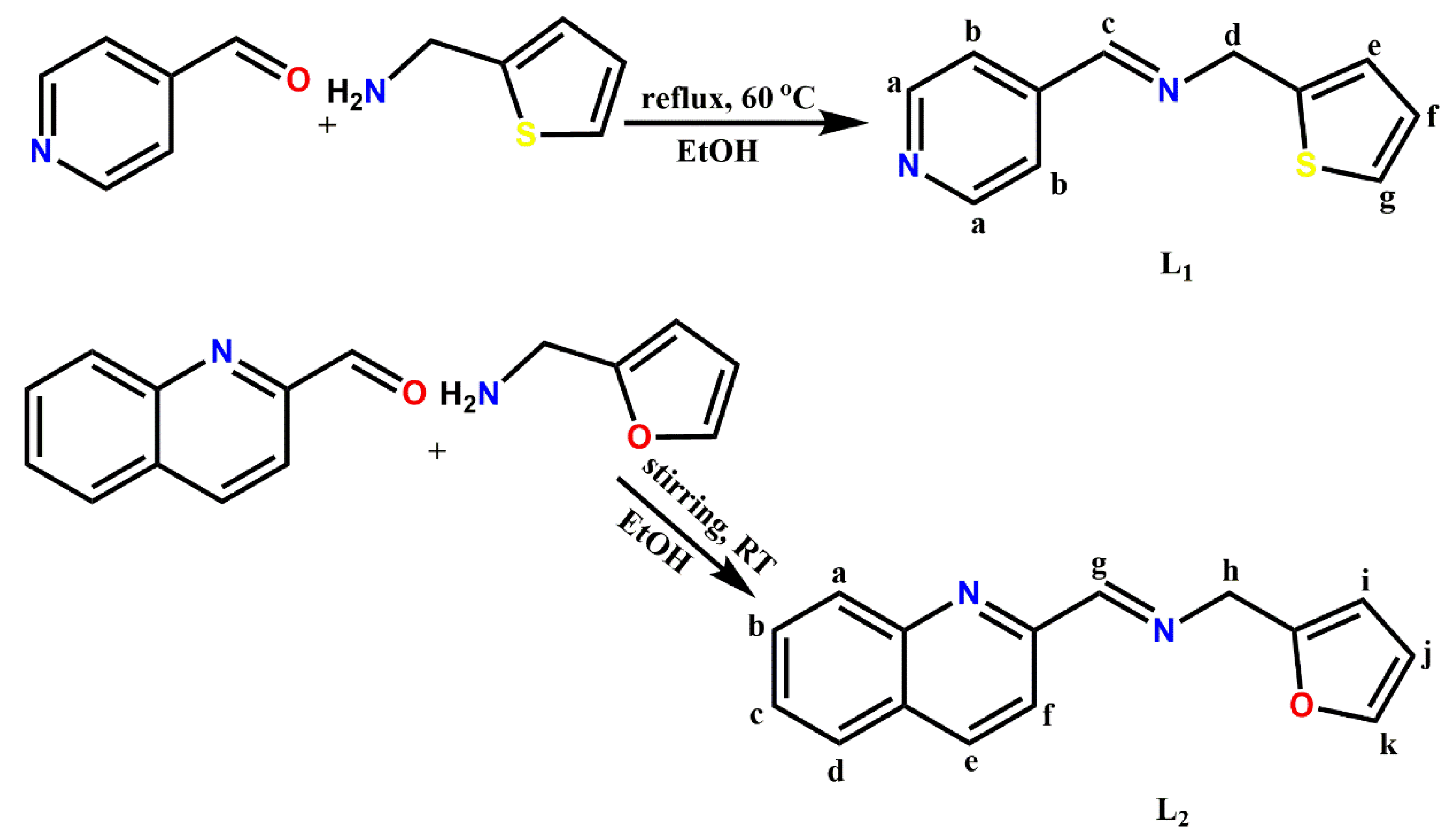
Imines are fundamental organic compounds used as synthetic intermediates and as ligands in coordination chemistry. They are also found to be important pharmacophores in various bioactive compounds. In this report, two Schiff bases were prepared using the traditional condensation of 4-pyridinecarboxaldehyde with 2-thiophenemethylamine and 2-quinolinecarboxaldehyde with furfurylamine to form (E)-1-(pyridin-4-yl)-N-(thiophen-2-ylmethyl)methanimine (L1) and (E)-N-(furan-2-ylmethyl)-1-(quinolin-2-yl)methanimine (L2) respectively. L1 and L2 were complexed with silver perchlorate in 2:1 [M:L] stoichiometry to obtain complexes 1 and 2, respectively. The crystal structures of 1 and 2 were unequivocally determined by single-crystal X-ray diffraction analysis. The resulting structures revealed 2 to be a four-coordinate as expected. In contrast, an unexpected chemoselective hydrolytic cleavage of one mole of the (CH=N) imine ligands occurred in complex 2 and, further, the amines (thiophenemethylamine) homo-coupled to form a new imine ligand derivative in situ (L1a) before coordinating to the Ag(I) center along with L1. This observation described an alternative synthetic route to be explored to synthesize a diverse range of imine derivatives, which involves the Ag(I)-promoted homo-coupling of amines. Herein, the crystal structures of Ag(I) complexes of pyridinyl [Ag(L1)(L1a)]ClO4 (1) and quinolinyl [Ag(L2)2]ClO4 (2) Schiff bases are presented.
Keywords:
Ag(I) complexes; thiophene; furan; pyridine; quinoline; homo-coupling amines; Schiff bases 1. Introduction
Schiff bases are one of the most widely used N-donor organic ligands. This can be attributed to their facile synthesis, high stability and high solubility in most organic solvents and their ability to coordinate to various transition metals and a wide range of applications [1,2]. The preparation of Schiff bases can proceed by the condensation reaction of carbonyl compounds with primary amines. Apart from the condensation reaction commonly used in the synthesis of Schiff bases, other synthetic methods have been reported, such as the reductive imination of nitro compounds [3], rebound hydrolysis [4], hydroamination of alkynes with anilines [5], oxidative coupling of alcohols and amines [6] and cross-coupling of amines [7,8].
An organic compound characterized by the presence of a pyridine or quinoline moiety with azomethine linkage to O- or S-heterocycle has been reported to exhibit a broad range of biological activities [9,10,11]. Their coordination to transition metals has been reported to enhance their biological activities. In this study, crystal structures of Ag(I) perchlorate complexes of pyridinyl (with thiophene moiety) and quinolinyl (with furan moiety) Schiff bases are reported.
2. Results
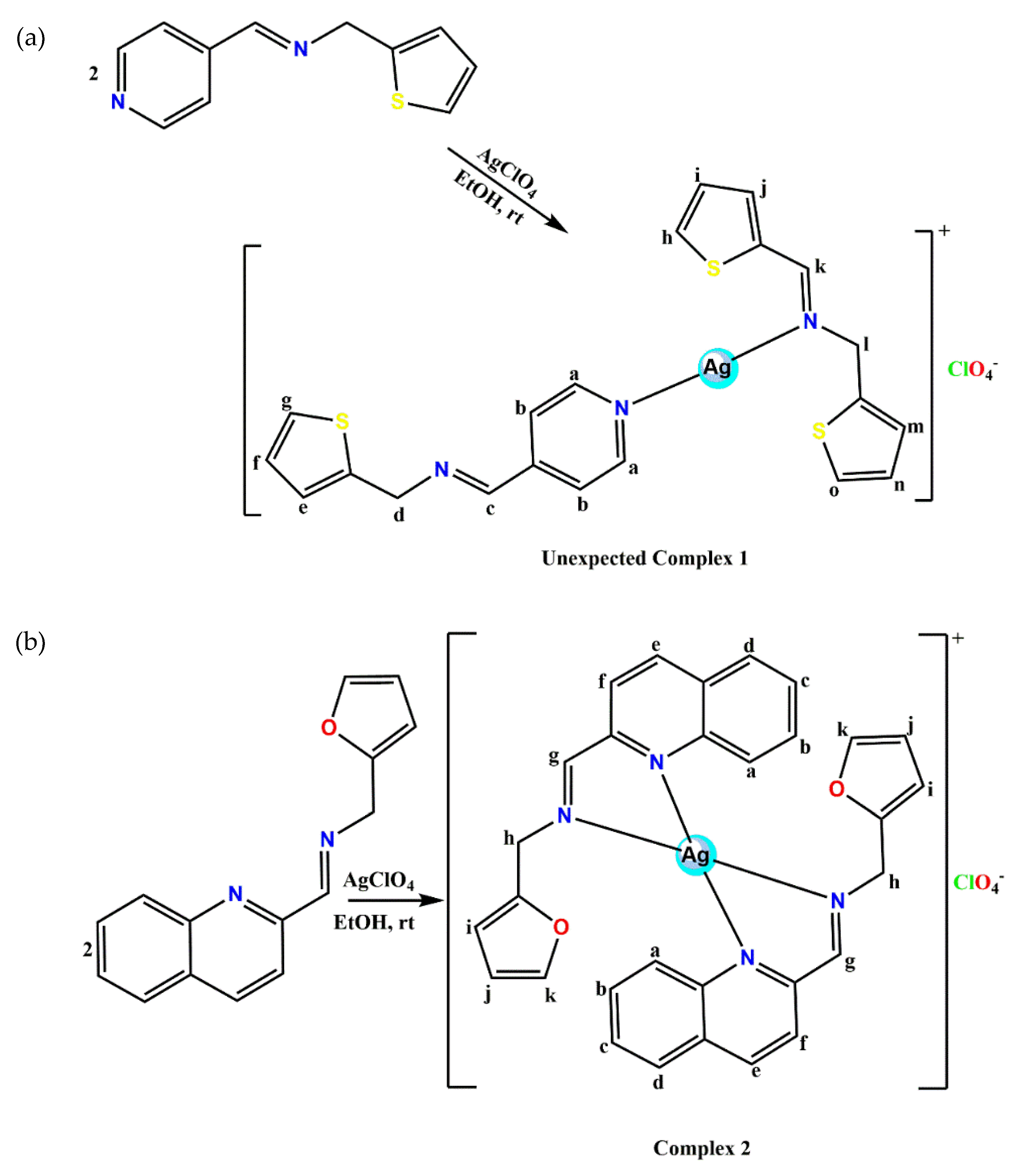
The unexpected thiophenemethylamine homo-coupling pyridinyl imine Ag(I) complexes (1) and quinolinyl imine Ag(I) complexes (2) were prepared by reaction of either L1 or L2 with silver perchlorate in 2:1 (L:M) stoichiometry. The four-coordinate complex 2 was isolated as proposed but in an attempt to complex L1 ((E)-1-(pyridin-4-yl)-N-(thiophen-2-ylmethyl)methanimine) with silver perchlorate in 2:1 (L1:M), a chemoselective hydrolytic cleavage of the (-CH=N) imine bond of one of the two ligands occurred. This is followed by instant cross-coupling of the thiophene amines in the presence of a Ag(I) ion to form a new imine ligand ((E)-1-(thiophen-2-yl)-N-(thiophen-2-ylmethyl)methanimine L1a). Afterward, L1 and L1a coordinated to the Ag(I) ion leading to a linear Ag(I) complex (1). This method of imine synthesis agrees with that from the literature [8], where transition metals act as a catalyst during the formation of imine from amines. The possibility of imine metathesis could be inconsequential since after the chemoselective hydrolytic cleavage of one molecule of the (CH=N) imine ligands, there seem to be no exchange equilibria of the R groups between the two molecules of the Schiff bases. The structures of 1 and 2 were confirmed by NMR, FT-IR, UV-vis, Mass spec, elemental analysis and X-ray diffraction.
The 1H NMR spectrum of 1 exhibits two singlets assigned to imine (CH=N) protons at 8.63 and 8.54 ppm signifying their different resonance. The signal of the -CH2 protons for the two ligands was seen as singlets at 4.91 and 5.01 ppm. The integration values and the significant downfield shifts in the newly formed (L1a) imine proton, the CH2 protons and the alpha proton on the pyridine ring relative to L1 resonance peaks (Figure S5) confirmed the confirmed the mode of coordination in complex 1 to be via the pyridinyl (Npy) and the imine nitrogen (Nim) atoms. In the 1H NMR spectrum of 2, the azomethine alpha protons and the alpha proton on the quinoline ring shifted downfield relative to the free L2 resonance peaks (Figure S4). This confirmed the coordination of complex 2 to be via the Nim and Nqy atoms in an N,N-bidentate manner. The coordination mode in complex 2 is similar to the reported four coordinate Ag(I) complexes [12]. Electronic absorption spectra of the complexes were recorded in acetonitrile at room temperature in the UV-Vis region. The absorption spectra of 1 (Figure S1) showed two absorption bands at 292 nm and 365 nm and red-shifted with respect to L1 absorption bands. These absorption bands are attributable to π–π* and n–π* transitions of the imine and pyridine ring. In 2, only one absorption band (Figure S2) at 313 nm assigned to π–π* transition was seen. Complex 2 red-shifted with respect to its free ligand L2, similar to reported quinolinyl Ag(I) complexes [13]. In the FT-IR spectroscopy study of 1 and 2 (Figures S13 and S14), a sharp absorption band assigned to imines at 1609 cm−1 and 1620 cm−1 respectively, were observed. These imine absorption bands shifted to lower frequencies with respect to their free ligands suggesting the coordination of the free ligands to Ag(I) ion. The calculated molecular formula of 1 [C21H19AgN3S3]+ and 2 [C30H24AgN4O2]+ agreed with their found molecular ion peak at m/z 518 and m/z 581 respectively obtained in the positive ion mode. The microanalyses of 1 and 2 are in good accordance with the reported structures.
Crystal Structures
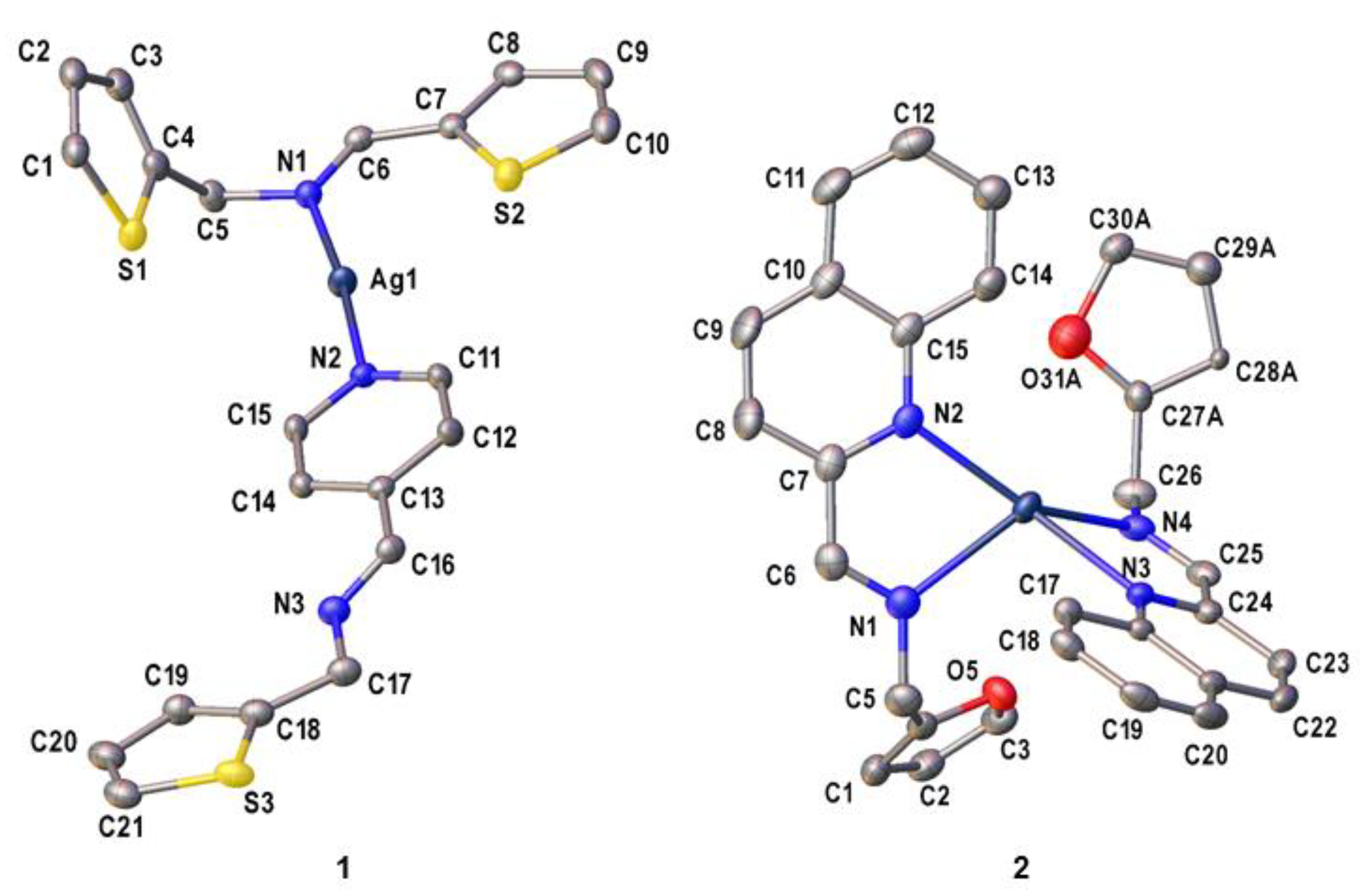
Crystals of 1 and 2 suitable for X-ray crystallography were obtained by diffusing hexane into dichloromethane solutions of 1 to obtain a yellow block of 1, while for 2, slow diffusion of toluene into dichloromethane solutions of 2 was done to obtain a brown needle crystal. The asymmetric unit of 1 and 2 consist of a cationic complex bearing one Ag(I) center and two imine ligand derivatives with one perchlorate counterion and a hydrate molecule as shown in Figure 1. Complex 1 has a mixed ligand system which comprises of L1 and L1a. Furthermore, L1 and L1a coordinate to the Ag(I) center via the pyridinyl (Npy) and the imine nitrogen (Nim) atoms, respectively. This results in a distorted linear geometry [14] around the metal center with an Npy—Ag—Nim bond angle of 174.31(8)°. Interestingly, intramolecular Ag1…S1 (3.2622(9) Å, symmetry code: x, y, z) and intermolecular Ag1…S3 (3.169(1) Å, symmetry code: 1-x, 2-y,2-z) interactions were found to be shorter than the sum of the van der Waals radii (3.52 Å) [15,16]. Unlike in 1, the Ag(I) center in 2 is coordinated to two identical (E)-N-(furan-2-ylmethyl)-1-(quinolin-2-yl)methanimine ligands which exhibit a κ2N,N’ coordination mode. The coordination environment around the metal center is occupied by two quinolinyl nitrogen (Nqy) and Nim atoms from separate ligands. Moreover, the τ4 value of complex 2 is equal to 0.83, indicating its distorted trigonal pyramidal geometry [17] with N—Ag—N bond angles ranging between 99.46(7)° and 143.77(8)°. Other selected bond parameters in 1 and 2 are listed in Table 1 and are comparable with those of closely related compounds in the literature [18,19].
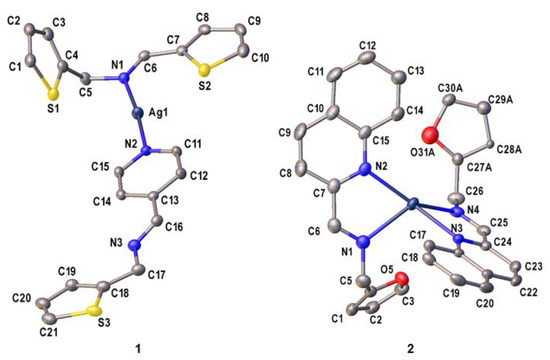
Figure 1.
The ORTEP diagrams of complexes 1 and 2 with the thermal ellipsoids drawn at the 50% probability level. The hydrate molecule in 1 including all hydrogen atoms and perchlorate anions in both complexes have been omitted for clarity.

Table 1.
Selected geometric parameters for complexes 1 and 2.
3. Materials and Methods
Ethanol 99.5% (Aldrich, St. Louis, Missouri, MO, USA), diethyl ether 99.8% (Aldrich, St. Louis, Missouri, MO, USA), DMSO-d6 99.8% (Merck, Darmstadt, Germany), 4-pyridinecarboxaldehyde 99% (Aldrich, St. Louis, Missouri, MO, USA), 2-quinolinecarboxaldehyde 99% (Aldrich, St. Louis, Missouri, MO, USA), 2-thiophenemethylamine > 92% (MerckDarmstadt, Germany), furan-2-ylmethanamine 99% (Aldrich, St. Louis, Missouri, MO, USA), dichloromethane 99% (Aldrich, St. Louis, Missouri, MO, USA), and nitrogen gas, 5.0 technical grade (Air flex Industrial Gases, Pietermaritzburg, Africa) were purchased from local suppliers. All chemicals were analytical grade and were used as received, while most of the solvents were dried using conventional techniques.
1H NMR and 13C NMR spectra were recorded on a BRUKER 400 MHz spectrometer in DMSO-d6 and acetone-d6. Chemical shift values are reported in parts per million (ppm) relative to the solvent residual peaks in DMSO-d6 and acetone-d6; 2.5 and 2.05 ppm respectively for 1H NMR and 39.5 and 29.4 ppm respectively for 13C NMR. The splitting patterns in 1H NMR spectra are reported as s for singlet, d for doublet, m for multiplet and J (the coupling constant is given in Hertz). The infrared spectra were recorded using a PerkinElmer Spectrum 100 FT-IR spectrometer, and the data are reported as percentage transmittances at the respective wavenumbers (cm−1), between 4000 and 650 cm−1. The mass spectra were recorded using the Shimadzu LCMS-2020 instrument with only molecular ions (M+) and major fragmentation peaks being reported with intensities quoted as percentages of the base peak. Elemental analyses were performed on Thermal-Scientific Flash 2000 CHNS/O analyzer. All melting points were determined using the Stuart Scientific melting point apparatus.
3.1. Synthesis of Pyridinyl (L1) and Quinolinyl (L2) Schiff bases
Ligands (E)-1-(pyridin-4-yl)-N-(thiophen-2-ylmethyl)methanimine (L1) and (E)-N-(furan-2-ylmethyl)-1-(quinolin-2-yl)methanimine (L2) (Scheme 1) were prepared using similar methods reported by our group [20,21].
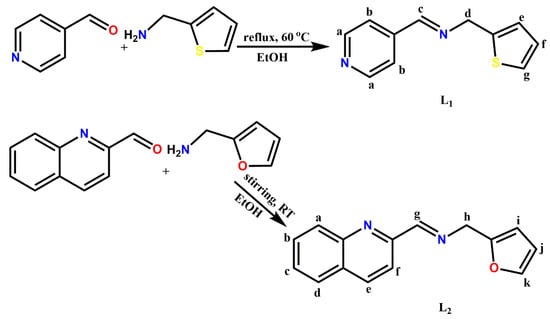
Scheme 1.
Synthesis of pyridinyl (L1) and quinolinyl (L2) Schiff bases.
Synthesis of L1: 4-pyridinecarboxaldehyde (1 mmol, 0.11 g, 0.09 mL) in anhydrous ethanol (10 mL) was added slowly to a stirred hot solution of 2-thiophenemethylamine (1 mmol, 0.11 g, 0.10 mL) in anhydrous ethanol (10 mL) in the presence of a few drops of glacial acetic acid. The reacting mixture was reflux at 80 °C for 4 h. The resulting reaction solution was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The yellow oil obtained was recrystallized from ethanol. Yield: 0.18 g, 89%, 1H-NMR (400 MHz, (CD3)2CO, δ ppm): 8.69 (2H, m, Hb-C5H4N), 8.48 (1H, s, Hc-CH=N-), 7.71 (2H, m, Ha-C5H4N), 7.36 (1H, s, Hg-C4H4S), 7.03 (1H, m, He-C4H4S), 7.00 (1H, m, Hf-C4H4S), 5.03 (2H, s, Hd-CH2). 13C-NMR (100 MHz, (CD3)2CO, 25 °C): δ = 156.24 (C6-C=N-), 151.04 (C5-C5H4N), 150.02 (C2 & C3-C5H4N), 140.56 (C8- C4H3S), 127.22 (C10-C4H3S), 125.17 (C9- C4H3S), 124.87 (C11-C4H3S), 121.66 (C1 & C4-C5H4N), 58.96 (C7-CH2). FT-IR (cm−1): (-C=N-) 1628, (pyridinyl) 1596, (CH2) 1411, (thiophene) 698. UV/Vis (CH3CN): λmax 287, 363 nm. MS: m/z Calcd. For [C11H10N2S]: 202.28; found [3L1 + 3MeOH]+: 705 (100%), [L1]+ 203 (69%), [L1 + MeOH]+ 33%.
Synthesis of L2: Ligand 2 was synthesized by the gradual addition of 2-quinolinecarboxaldehyde (1 mmol, 0.16 g) dissolved in anhydrous ethanol (10 mL) to a solution of furfurylamine (1 mmol, 0.10g, 0.09 mL), respectively, in anhydrous ethanol (10 mL) in the presence of glacial acetic acid. The reactant mixture was subjected to constant stirring at ambient temperature for 6 h. Afterward, the resulting solution was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to obtain a yellow oil product. The oil obtained was recrystallized from ethanol. Yield: 0.21 g, 88%, 1H-NMR (400 MHz, (CD3)2CO, δ ppm): 8.57 (1H, d, J = 0.82 Hz, Hg-CH=N-), 8.37 (1H, d, J = 8.63 Hz, Hd-C9H6N), 8.16 (1H, d, J = 8.55 Hz, Hd-C9H6N), 8.09 (1H, d, J = 9.56 Hz, Ha-C9H6N), 7.99 (1H, m, Hf-C9H6N), 7.80 (1H, m, Hb-C9H6N), 7.65 (1H, m, Hc-C9H6N), 7.53 (1H, m, Hk-C4H3O), 6.41 (2H, m, Hj, i-C4H3O), 4.90 (2H, d, J = 1.31 Hz, Hh-CH2). 13C-NMR (100 MHz, (CD3)2CO, 25 °C): δ = 153.70 (C9-C9H6N), 152.17 (C10-C=N-), 149.20 (C12-C4H3O), 147.86 (C1-C9H6N), 142.26 (C15-C4H3O), 137.63 (C7-C9H6N), 130.92 (C6-C9H6N), 130.51 (C3-C9H6N), 129.66 (C2-C9H6N), 128.69 (C5-C9H6N), 128.22 (C4-C9H6N), 121.62 (C8-C9H6N), 110.97 (C14-C4H3O), 108.15 (C13-C4H3O), 48.88 (C11-CH2). FT-IR (cm−1): (Ar-CH) 3055, (-C=N-) 1641, (quinolinyl) 1594, (CH2) 1425, (furan), 734. UV/Vis (CH3CN): λmax 295 nm. MS: m/z Calcd. For [C15H12N2S]: 252.34; found [2L2 + CH3CN]+: 547 (100%), [2L2 + Na + 2H)]+ 531 (36%), [L2 + 2CH3CN + Na + 2H] 360 (25%).
3.2. Synthesis of Pyridinyl and Quinolinyl Ag(I) Complexes
A slightly modified procedure from our group was also used [22,23] in the synthesis of the Ag(I) complexes as shown in Scheme 2a,b.
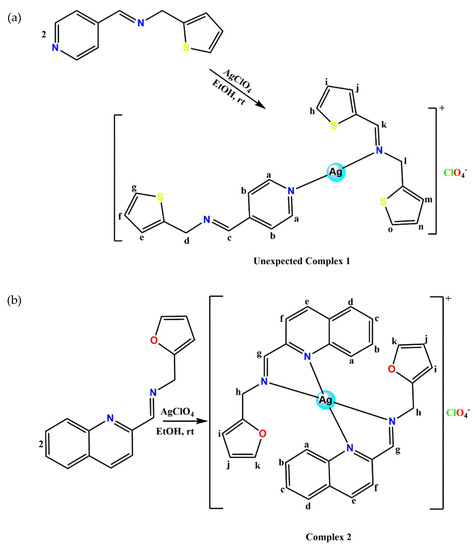
Scheme 2.
(a) Synthesis of Ag(I) complex of imine derivatives (1); (b) Synthesis of Ag(I) complex of quinolinyl Schiff bases (2).
Complex 1: L1 (5 mmol, 1.01 g) dissolved in anhydrous ethanol (15 mL) was added dropwise to an ethanolic (15 mL) solution of silver perchlorate (2.5 mmol, 0.52 g) under constant stirring in the dark. The reaction was carried out under nitrogen at ambient temperature for 6 h. The resulting solid precipitate was isolated using a vacuum filter. Afterwards, the precipitate was washed with cold ethanol (10 mL X2) followed by cold ether (10 mL X2) and dried in vacuo. The yellow precipitate isolated was recrystallized by diffusing hexane into dichloromethane solutions of the complexes to obtain a yellow block crystal suitable for X-ray crystallography. Yield: 0.79 g, 52%, M.pt. 161–162 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 8.69 (2H, m, Hb-C5H4N), 8.63 (1H, s, Hc-C=N), 8.54 (1H, s, Hl-C=N), 7.75 (2H, m, Ha-C5H4N), 7.55 (1H, m, Hm-C4H3S), 7.43 (3H, m, Hg, h, o-C4H3S), 7.27 (3H, m, Hf, i, n-C4H3S), 7.02 (2H, m, He, j-C4H3S), 5.01 (2H, s, Hd-CH2), 4.91 (2H, s, Hk-CH2). 13C-NMR (100 MHz, DMSO-d6, 25 °C): δ = 160.89 (C16-C=N-), 156.47 (C6-C=N-), 150.70 (C7-C5H4N), 150.12 (C10-C5H4N), 150.08 (C11-C5H4N), 139.05 (C4-C4H3S), 138.49 (C18-C4H3S), 127.97 (C14-C4H3S), 127.58 (C13-C4H3S), 127.02 (C20-C4H3S), 126.99 (C2-C4H3S), 126.50 (C3 & C19-C4H3S), 125.99 (C12-C4H3S), 125.35 (C1 & C21-C4H3S), 125.33 (C15-C4H3S), 125.29 (C8 & C9-C5H4N), 58.40 (C5-CH2), 57.89 (C17-CH2). FT-IR (cm−1): (pyridinyl) 1609, (-C=N) 1609, (CH2) 1424, (thiophene) 706. UV/Vis (CH3CN): λmax 292, 365 nm. MS: m/z Calcd. For [C21H19AgN3S3]: 517.45; found [Ag(L1)2 + 3CH3CN]+: 641 (100%). Anal. Calcd. (%) for [C21H19AgClN3O4S3]: C, 40.89; H, 3.10; N, 6.81; found (%): C, 40.72; H, 3.06; N, 6.75.
Complex 2: L2 (2 mmol, 0.51 g) dissolved in anhydrous ethanol (10 mL) was added dropwise to an ethanolic (10 mL) solution of silver perchlorate (1 mmol, 0.21 g) under constant stirring in the dark. The reaction was carried out under nitrogen at ambient temperature for 6 h. A brown solid product was obtained and isolated using a vacuum filter. Afterwards, the brown solid product was washed with cold ethanol twice followed by cold ether (10 mL X2) and dried in vacuo. The brown solid was recrystallized by diffusing toluene into dichloromethane solutions of the complexes to obtain a brown needle crystal suitable for X-ray crystallography. Yield: 0.63 g, 93%, Melting point: 149–151 °C, 1H-NMR (400 MHz, (CD3)2CO, δ ppm): 8.90 (2H, s, Hg-CH=N-), 8.70 (2H, d, J = 8.44 Hz, He-C9H6N), 8.11 (4H, m, Hd, a-C9H6N), 8.03 (2H, d, J = 8.63 Hz, Hf-C9H6N), 7.87 (2H, t, Hb-C9H6N), 7.74 (2H, t, Hc-C9H6N), 7.56 (2H, d, J = 1.13 Hz, Hk-C4H3O), 6.51 (2H, m, Hj-C4H3O), 6.45 (1H, m, Hi-C4H3O), 6.39 (1H, t, J = 2.47 Hz, Hi-C4H3O), 5.01 (2H, s, m, Hh-CH2), 4.08 (2H, s, Hh-CH2). 13C-NMR (100 MHz, (CD3)2CO, 25 °C): δ = 163.47 (C10-C=N-), 150.93 (C9-C9H6N), 149.98 (C12-C4H3O), 145.91 (C1-C9H6N), 143.09 (C15-C4H3O), 139.46 (C7-C9H6N-), 131.67 (C6-C9H6N), 129.80 (C3-C9H6N), 129.31 (C2-C9H6N), 128.75 (C5-C9H6N), 128.34 (C4-C9H6N), 123.36 (C8-C9H6N), 110.63 (C14-C4H3O), 108.78 (C13-C4H3O), 55.82 (C11-CH2). FT-IR (cm−1): (-C=N-) 1620, (quinolinyl) 1586, (CH2) 1433, (furan) 752. UV/Vis (CH3CN): λmax 313 nm. MS: m/z Calcd. for [C30H24AgN4O2]: 580.42; found [Ag(L2)2 + 2CH3CN + K]+: 705 (100%). Anal. Calcd. (%) for [C30H24AgClN4O6]: C, 53.00; H, 3.56; N, 8.24; found (%): C, 53.11; H, 3.32; N, 8.02.
3.3. X-ray Crystallography
Crystal evaluation and data collection of the complexes 1 and 2 were recorded on a Bruker Apex Duo diffractometer equipped with an Oxford Instruments Cryojet operating at 100 (2) K and an Incoatec microsource operating at 30 W power. The data were collected with Mo Kα (λ = 0.71073 Å) radiation at a crystal-to-detector distance of 50 mm using omega and phi scans. The data were reduced with the program SAINT [24] using outlier rejection, scan speed scaling, as well as standard Lorentz and polarization correction factors. A SADABS [25] semi-empirical multi-scan absorption correction was applied to the data. The structures of complexes 1 and 2 were solved by the direct method using the SHELXS [26] program and refined. The visual crystal structure information was performed using ORTEP-3 [27], system software. Non-hydrogen atoms were first refined isotropically and then by anisotropic refinement with a full-matrix least-squares method based on F2 using SHELXL [28]. All hydrogen atoms were positioned geometrically, allowed to ride on their parent atoms, and refined isotropically. The crystallographic data and structure refinement parameters of complexes 1 and 2 are given in Table 2. The two thiophenyl rings in 1 were found to be disordered over two positions. PART instructions were used to resolve the disorder, with the major components having 0.6 and 0.9 site occupancy factors. In 2, the furanyl ring and the perchlorate anion exhibited disorder over two positions. PART instructions were used to model the disorder and the major component had 0.722(8) site occupancy factor.

Table 2.
Crystal data and structure refinement for complexes 1 and 2.
4. Conclusions
The conversion of amines to imines in the presence of Ag(I) ion as a catalyst, without being under aerobic conditions or any other additives, has been demonstrated herein. The homo-coupling of thiophene amines derived from the selective hydrolytic cleavage of an imine bond to form a new imine yielded a linear Ag(I) complex of imine derivatives (1). In complex 2, the quinolinyl Schiff base adopts a pseudo-trigonal pyramidal geometry around the Ag(I) center. The spectroscopic data confirmed the structures of complexes 1 and 2.
The comparison of the crystal structures of 1 and 2 revealed their different structural features. Interestingly, both complexes were prepared from silver perchlorate complexes of either pyridinyl (L1) or quinolinyl (L2) Schiff bases, with each ligand having a methylene linker to a five-member heterocyclic moiety. However, the conjugated ring and the position of the N atom in 2 must have influenced its stability in the presence of the Ag(I) ion. As such, L2 coordinated as expected in an N,N-bidentate manner (2). Considering the cost-effectiveness of anilines and the availability of silver salts, this report has proven a facile reaction route at ambient temperature and mild reaction conditions to explore the synthesis of a diverse range of silver-promoted imine derivatives.
Supplementary Materials
The following are available online. Figures S1 and S2: Electronic absorption spectra of L1, L2, complexes 1 and 2, Figures S3–S6: 1H NMR spectra of L1 and L2 and 1 and 2, Figures S3–S6: 13C NMR spectra of L1 and L2 and 1 and 2, Figures S7–S10: IR spectra of L1 and L2 and 1 and 2, Figure S11–S14: Mass Spec. spectra of L1 and L2 and 1 and 2: Figures S15–S18.
Author Contributions
B.O. conceived and designed the experiments; A.A.A. carried out the synthesis, crystal growth and characterized the reported structures. A.A.A. drafted the manuscript; S.J.Z. reported the crystal structures; B.O. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of South Africa (Grantnumber: 119342) and the APC was funded by the National Research Foundation of South Africa.
Data Availability Statement
CCDC 2083791 and 2083792 contain the supplementary crystallographic data for complexes 1 and 2, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 14 May 2021), or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44)1223-336-033; or via email: deposit@ccdc.cam.ac.uk.
Acknowledgments
Adesola A. Adeleke acknowledges Olabisi Onabanjo University, Ago-Iwoye, for granting her study leave to pursue the programme at the University of KwaZulu-Natal. The authors would like to thank the University of KwaZulu-Natal and the National Research Foundation of South Africa (Grant number: 119342) for their financial assistance of Adesola A. Adeleke.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prakash, A.; Adhikari, D. Application of Schiff bases and their metal complexes—A Review. Int. J. Chem. Tech. Res. 2011, 3, 1891–1896. [Google Scholar]
- Rudrapal, M. Chemistry and Biological Importance of Heterocyclic Schiff’s Bases. Int. Res. J. Pure Appl. Chem. 2014, 3, 232–249. [Google Scholar] [CrossRef]
- Huang, J.; Yu, L.; He, L.; Liu, Y.-M.; Cao, Y.; Fan, K.-N. Direct one-pot reductive imination of nitroarenes using aldehydes and carbon monoxide by titania supported gold nanoparticles at room temperature. Green Chem. 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Shao, Y.; Xu, G.; Zhang, X.; Tang, S.; Sun, J. Rhodium-Catalyzed C horizontal lineN Bond Formation through a Rebound Hydrolysis Mechanism and Application in beta-Lactam Synthesis. Org. Lett. 2019, 21, 4124–4127. [Google Scholar] [CrossRef]
- Casnati, A.; Voronov, A.; Ferrari, D.G.; Mancuso, R.; Gabriele, B.; Motti, E.; Della Ca’, N. PdI2 as a Simple and Efficient Catalyst for the Hydroamination of Arylacetylenes with Anilines. Catalysts 2020, 10, 176. [Google Scholar] [CrossRef]
- Wu, S.; Sun, W.; Chen, J.; Zhao, J.; Cao, Q.; Fang, W.; Zhao, Q. Efficient imine synthesis from oxidative coupling of alcohols and amines under air atmosphere catalysed by Zn-doped Al2O3 supported Au nanoparticles. J. Catal. 2019, 377, 110–121. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, B.; Mullick, K.; Kuo, C.-H.; Poyraz, A.S.; Suib, S.L. Aerobic Oxidation of Amines to Imines by Cesium-Promoted Mesoporous Manganese Oxide. ACS Catalysis 2015, 5, 4394–4403. [Google Scholar] [CrossRef]
- Largeron, M. Protocols for the Catalytic Oxidation of Primary Amines to Imines. Eur. J. Org. Chem. 2013, 2013, 5225–5235. [Google Scholar] [CrossRef]
- Alam, M.; Sarkar, P.; Husain, A.; Marella, A.; Zaman, M.S.; Akhter, M.; Shaharyar, M.; Alam, O.; Azam, F. Synthesis of quinoline attached-furan-2(3H)-ones having anti-inflammatory and antibacterial properties with reduced gastro-intestinal toxicity and lipid peroxidation. J. Serb. Chem. Soc. 2011, 76, 1617–1626. [Google Scholar] [CrossRef]
- Shakir, M.; Hanif, S.; Sherwani, M.A.; Mohammad, O.; Al-Resayes, S.I. Pharmacologically significant complexes of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) of novel Schiff base ligand, (E)-N-(furan-2-yl methylene) quinolin-8-amine: Synthesis, spectral, XRD, SEM, antimicrobial, antioxidant and in vitro cytotoxic studies. J. Mol. Struct. 2015, 1092, 143–159. [Google Scholar] [CrossRef]
- Alturiqi, A.S.; Alaghaz, A.-N.M.A.; Ammar, R.A.; Zayed, M.E. Synthesis, Spectral Characterization, and Thermal and Cytotoxicity Studies of Cr(III), Ru(III), Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) Complexes of Schiff Base Derived from 5-Hydroxymethylfuran-2-carbaldehyde. J. Chem. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Stenger-Smith, J.; Chakraborty, I.; Sameera, W.M.C.; Mascharak, P.K. Antimicrobial silver(I) complexes derived from aryl-benzothiazoles as turn-on sensors: Syntheses, properties and density functional studies. Inorg. Chim. Acta 2018, 471, 326–335. [Google Scholar] [CrossRef]
- Jimenez, J.; Chakraborty, I.; Rojas-Andrade, M.; Mascharak, P.K. Silver complexes of ligands derived from adamantylamines: Water-soluble silver-donating compounds with antibacterial properties. J. Inorg. Biochem. 2017, 168, 13–17. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, L.-L.; Fan, J.; Chen, K.; Sun, W.-Y. Silver(I) complexes of 4-(2-oxazolinyl)pyridine: Counteranion dependent structural diversity. Polyhedron 2012, 46, 16–24. [Google Scholar] [CrossRef]
- Bondi, A.V. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, W.; Wei, D.; Chen, J.-H.; Ng, S.W.; Yang, G. Adducts of triangular silver (i) 3, 5-bis (trifluoromethyl) pyrazolate with thiophene derivatives: A weak interaction model of desulfurization. Dalton Trans. 2019, 48, 16162–16166. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, tau4. Dalton Trans. 2007, 9, 955–64. [Google Scholar] [CrossRef]
- Sun, W.-H.; Zhang, T.; Wang, L.; Chen, Y.; Froehlich, R. Supramolecular helical architecture assembled by double-helical [Ag2L2] units. J. Organomet. Chem. 2004, 689, 43–49. [Google Scholar] [CrossRef]
- Tatikonda, R.; Bulatov, E.; Kalenius, E.; Haukka, M. Construction of Coordination Polymers from Semirigid Ditopic 2,2′-Biimidazole Derivatives: Synthesis, Crystal Structures, and Characterization. Cryst. Growth Des. 2017, 17, 5918–5926. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Zamisa, S.J.; Omondi, B. Crystal structure of 4-(1-phenylimidazo [1,5-a]pyridin-3-yl)benzoic acid (C20H14N2O2). Z. Krist. N. Cryst. Struct. 2019, 234, 1157–1159. [Google Scholar]
- Adeleke, A.A.; Zamisa, S.J.; Omondi, B. Crystal structure of dichlorido-bis((E)-2-((pyridin-4-ylmethylene)amino)phenol)zinc(II), C24H20Cl2N4O2Zn. Z. Kristallogr. NCS 2020, 235, 625–628. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Zamisa, S.J.; Islam, M.S.; Olofinsan, K.; Salau, V.F.; Mocktar, C.; Omondi, B. Quinoline Functionalized Schiff Base Silver (I) Complexes: Interactions with Biomolecules and In Vitro Cytotoxicity, Antioxidant and Antimicrobial Activities. Molecules 2021, 26, 1205. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, A.A.; Islam, M.S.; Sanni, O.; Mocktar, C.; Zamisa, S.J.; Omondi, B. Aryl variation and anion effect on CT-DNA binding and in vitro biological studies of pyridinyl Ag(I) complexes. J. Inorg. Biochem. 2021, 214, 1–17. [Google Scholar] [CrossRef]
- SAINT, B. Data Reduction Software; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker, A. Saint and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. Crystal structure solution with ShelXT. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L. WinGX and ORTEP for Windows: An. update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).