Oxygenated Analogues of Santacruzamate A
Abstract
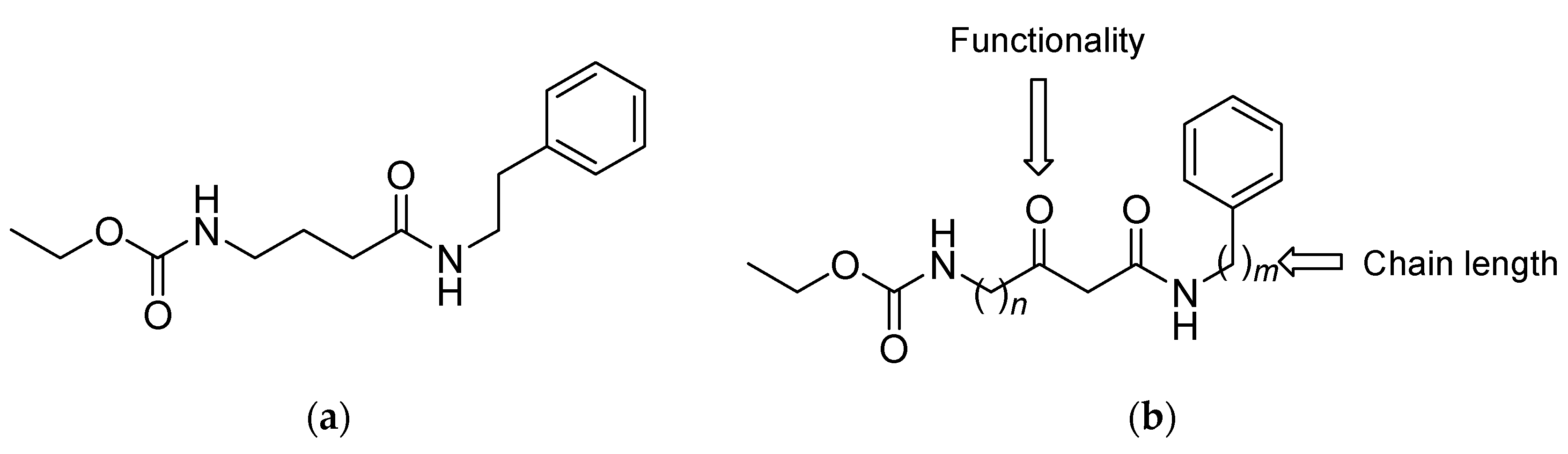
1. Introduction
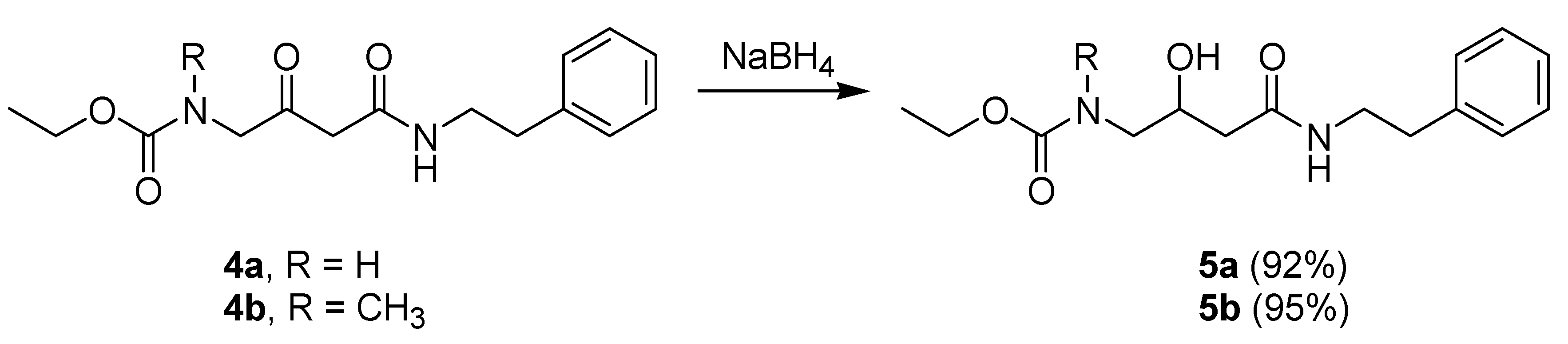
2. Results
3. Materials and Methods
Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavlik, C.M.; Wong, C.Y.B.; Ononye, S.; Lopez, D.D.; Engene, N.; McPhail, K.L.; Gerwick, W.H.; Balunas, M.J. Santacruzamate A, a Potent and Selective Histone Deacetylase Inhibitor from the Panamanian Marine Cyanobacterium cf. Symploca sp. J. Nat. Prod. 2013, 76, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A. Discovery and development of SAHA as an anticancer agent. Oncogene 2007, 26, 1351–1356. [Google Scholar] [CrossRef]
- Gromek, S.M.; deMayo, J.A.; Maxwell, A.T.; West, A.M.; Pavlik, C.M.; Zhao, Z.; Li, J.; Wiemer, A.J.; Zweifach, A.; Balunas, M.J. Synthesis and biological evaluation of santacruzamate A analogues for anti-proliferative and immunomodulatory activity. Bioorg. Med. Chem. 2016, 24, 5183–5196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, W.; Ma, M.; Liao, J.; Ganesan, A.; Hu, Y.; Wen, S.; Huang, P. Synthesis and biological evaluation of santacruzamate A and analogs as potential anticancer agents. RSC Adv. 2015, 5, 1109–1112. [Google Scholar] [CrossRef]
- Randino, R.; Gazzerro, P.; Mazitschek, R.; Rodriquez, M. Synthesis and biological evaluation of Santacruzamate-A based analogues. Bioorg. Med. Chem. 2017, 25, 6486–6491. [Google Scholar] [CrossRef] [PubMed]
- Krieger, V.; Hamacher, A.; Gertzen, C.G.W.; Senger, J.; Zwinderman, M.R.H.; Marek, M.; Romier, C.; Dekker, F.J.; Kurz, T.; Jung, M.; et al. Design, Multicomponent Synthesis, and Anticancer Activity of a Focused Histone Deacetylase (HDAC) Inhibitor Library with Peptoid-Based Cap Groups. J. Med. Chem. 2017, 60, 5493–5506. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.N.; Evangelista, F.C.G.; Seckler, D.; Marques, D.R.; Freitas, T.R.; Nunes, R.R.; Oliveira, J.T.; Ribeiro, R.I.M.A.; Santos, H.B.; Thomé, R.G.; et al. Synthesis, cytotoxic activity, and mode of action of new Santacruzamate A analogs. Med. Chem. Res. 2018, 27, 2397–2413. [Google Scholar] [CrossRef]
- Balunas, M.J.; Pavlik, C.M.; Gerwick, G.H. Santacruzamate A compositions and analogs and methods of use. Patent WO2014018913A2, 30 January 2014. Available online: https://patents.google.com/patent/WO2014018913A2/en. (accessed on 1 January 2021).
- Angelov, P. Enamine-Based Domino Strategy for C-Acylation/Deacetylation of Acetoacetamides: A Practical Synthesis of β-Keto Amides. Synlett 2010, 1273–1275. [Google Scholar] [CrossRef]
- Yanev, P.; Angelov, P. Synthesis of functionalised β-keto amides by aminoacylation/domino fragmentation of β-enamino amides. Beilstein J. Org. Chem. 2018, 14, 2602–2606. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, T.; Hansen, H.F.; Ørum, H.; Koch, T. PNA synthesis using a novel Boc/acyl protecting group strategy. J. Pept. Sci. 2001, 7, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Clemens, R.J.; Hyatt, J.A. Acetoacetylation with 2,2,6-trimethyl-4H-1,3-dioxin-4-one: A convenient alternative to diketene. J. Org. Chem. 1985, 50, 2431–2435. [Google Scholar] [CrossRef]
- Witzeman, J.S.; Nottingham, W.D. Transacetoacetylation with tert-butyl acetoacetate: Synthetic applications. J. Org. Chem. 1991, 56, 1713–1718. [Google Scholar] [CrossRef]

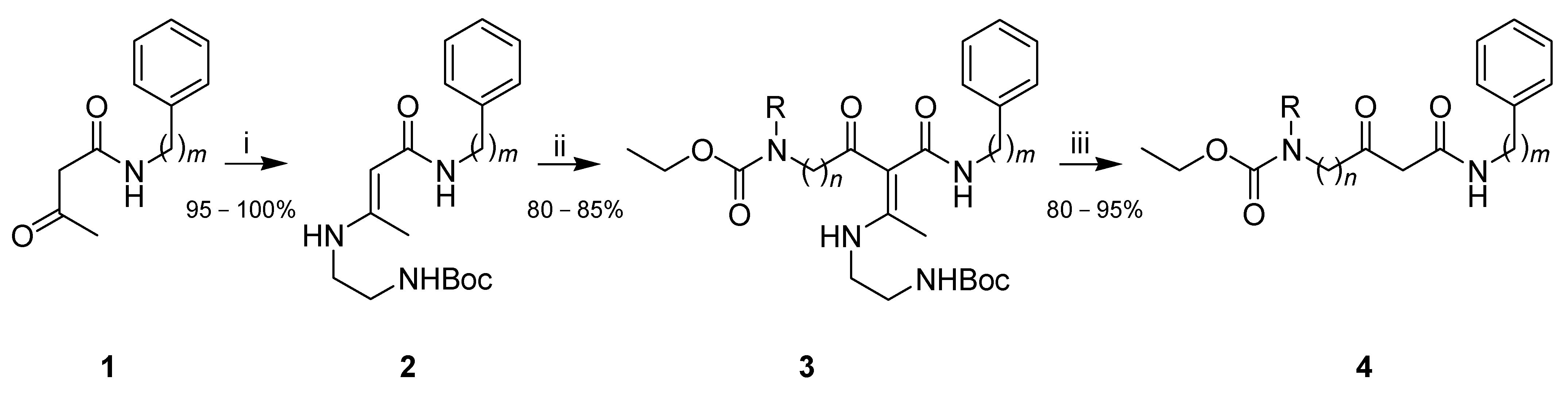
| 4 | n | m | R | Yield (%) 1 |
|---|---|---|---|---|
| a | 1 | 2 | H | 68 |
| b | 1 | 2 | CH3 | 76 |
| c | 2 | 1 | H | 71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelov, P.; Manolov, S.; Yanev, P.; Naydenov, M. Oxygenated Analogues of Santacruzamate A. Molbank 2021, 2021, M1188. https://doi.org/10.3390/M1188
Angelov P, Manolov S, Yanev P, Naydenov M. Oxygenated Analogues of Santacruzamate A. Molbank. 2021; 2021(1):M1188. https://doi.org/10.3390/M1188
Chicago/Turabian StyleAngelov, Plamen, Stanimir Manolov, Pavel Yanev, and Mladen Naydenov. 2021. "Oxygenated Analogues of Santacruzamate A" Molbank 2021, no. 1: M1188. https://doi.org/10.3390/M1188
APA StyleAngelov, P., Manolov, S., Yanev, P., & Naydenov, M. (2021). Oxygenated Analogues of Santacruzamate A. Molbank, 2021(1), M1188. https://doi.org/10.3390/M1188







