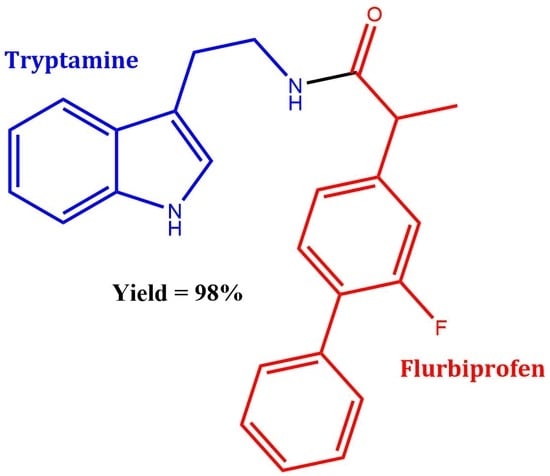
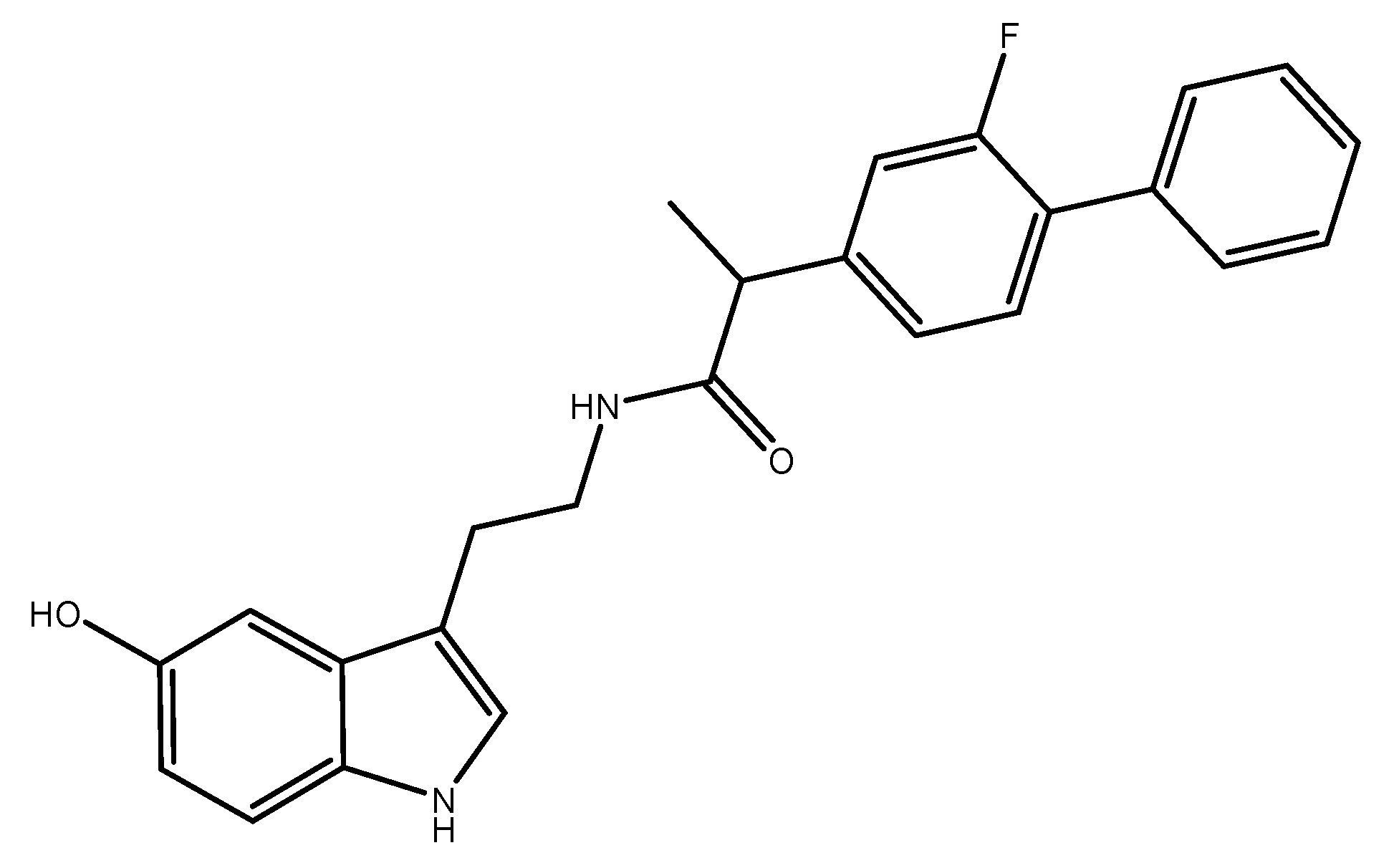
N-(2-(1H-Indol-3-yl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide
Abstract
1. Introduction
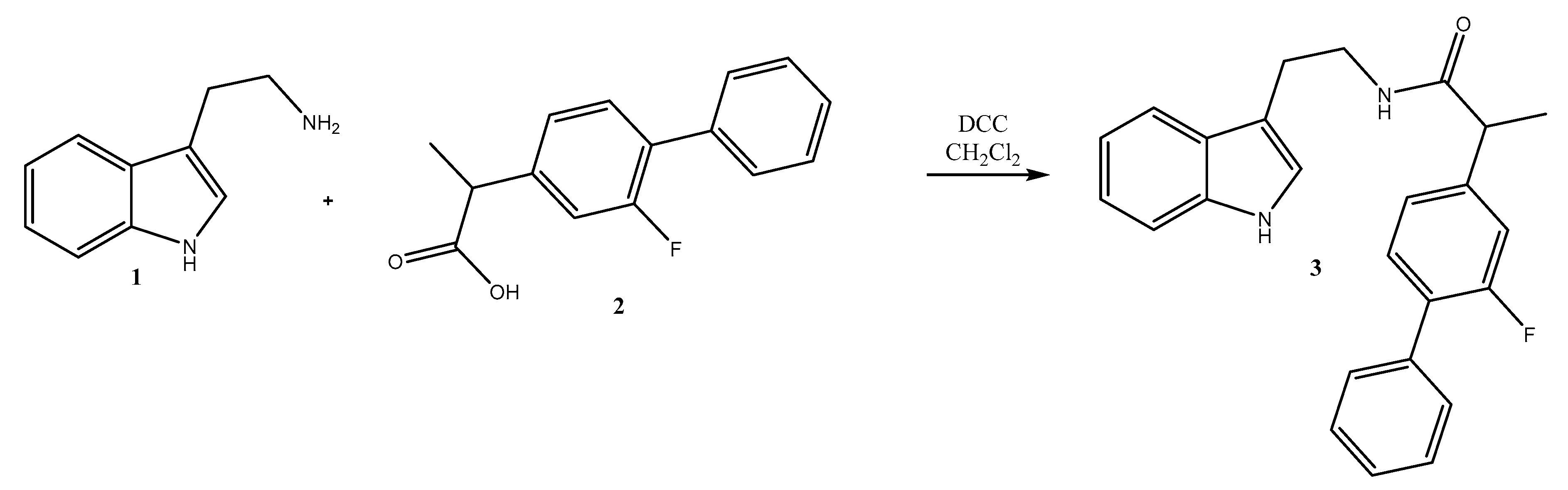
2. Results
3. Materials and Methods
Synthesis of N-(2-(1H-Indol-3-yl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 3
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Song, Q.; Cao, Z.; Yu, G.; Liu, Z.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of flurbiprofen-clioquinol hybrids as multitarget-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2020, 28, 115374. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Vergoten, G. Flurbiprofen as a biphenyl scaffold for the design of small molecules binding to PD-L1 protein dimer. Biochem. Pharmacol. 2020, 178, 114042. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Shallom, S.; Carlton, J.; Salzberg, S.; Nene, V.; Shoaibi, A.; Ciecko, A.; Lynn, J.; Rizzo, M.; Weaver, B.; et al. Sequence of Plasmodium falciparum chromosomes 2, 10, 11 and 14. Nature 2002, 419, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Bélard, S.; Ramharter, M. DSM265: A novel drug for single-dose cure of Plasmodium falciparum malaria. Lancet Infect. Dis. 2018, 18, 819–820. [Google Scholar] [CrossRef]
- Bartlett, R.R.; Brendel, S.; Zielinski, T.; Schorlemmer, H.-U. Leflunomide, an immunorestoring drug for the therapy of autoimmune disorders, especially rheumatoid arthritis. Transplant. Proc. 1996, 28, 3074–3078. [Google Scholar] [PubMed]
- Dexter, D.; Hesson, D.; Ardecky, R.; Rao, G.; Tippett, D.; Dusak, B.; Paull, K.; Plowman, J.; DeLarco, B.; Narayanan, V.; et al. Activity of a novel 4-quinolinecarboxylic acid, NSC 368390 [6-fluoro-2-(2’-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt], against experimental tumors. Cancer Res. 1985, 45, 5563–5568. [Google Scholar] [PubMed]
- Xiong, R.; Zhang, L.; Li, S.; Sun, Y.; Ding, M.; Wang, Y.; Zhao, Y.; Wu, Y.; Shamg, W.; Jiang, X.; et al. Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. Protein Cell 2020, 11, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Smart, B. Fluorine substituent effects (on bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef]
- Rose, T.M.; Reilly, C.A.; Deering-Rice, C.E.; Brewster, C.; Brewster, C. Inhibition of FAAH, TRPV1, and COX2 by NSAID-serotonin conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 5695–5698. [Google Scholar] [CrossRef]
- Trabocchi, A.; Mannino, C.; Machetti, F.; Bernardis, D.F.; Arancia, S.; Cauda, R.; Cassone, A.; Guarna, A. Identification of inhibitors of drug-resistant Candida albicans strains from a library of bicyclic peptidomimetic compounds. J. Med. Chem. 2010, 53, 2502–2509. [Google Scholar] [CrossRef]
- Brown, A.; Rees, D.; Rankovic, Z.; Morphy, R. Synthesis of tertiary amines using a polystyrene (REM) resin. J. Am. Chem. Soc. 1997, 119, 3288–3295. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D. N-(2-(1H-Indol-3-yl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide. Molbank 2021, 2021, M1177. https://doi.org/10.3390/M1177
Manolov S, Ivanov I, Bojilov D. N-(2-(1H-Indol-3-yl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide. Molbank. 2021; 2021(1):M1177. https://doi.org/10.3390/M1177
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, and Dimitar Bojilov. 2021. "N-(2-(1H-Indol-3-yl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide" Molbank 2021, no. 1: M1177. https://doi.org/10.3390/M1177
APA StyleManolov, S., Ivanov, I., & Bojilov, D. (2021). N-(2-(1H-Indol-3-yl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide. Molbank, 2021(1), M1177. https://doi.org/10.3390/M1177