4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline
Abstract
:1. Introduction
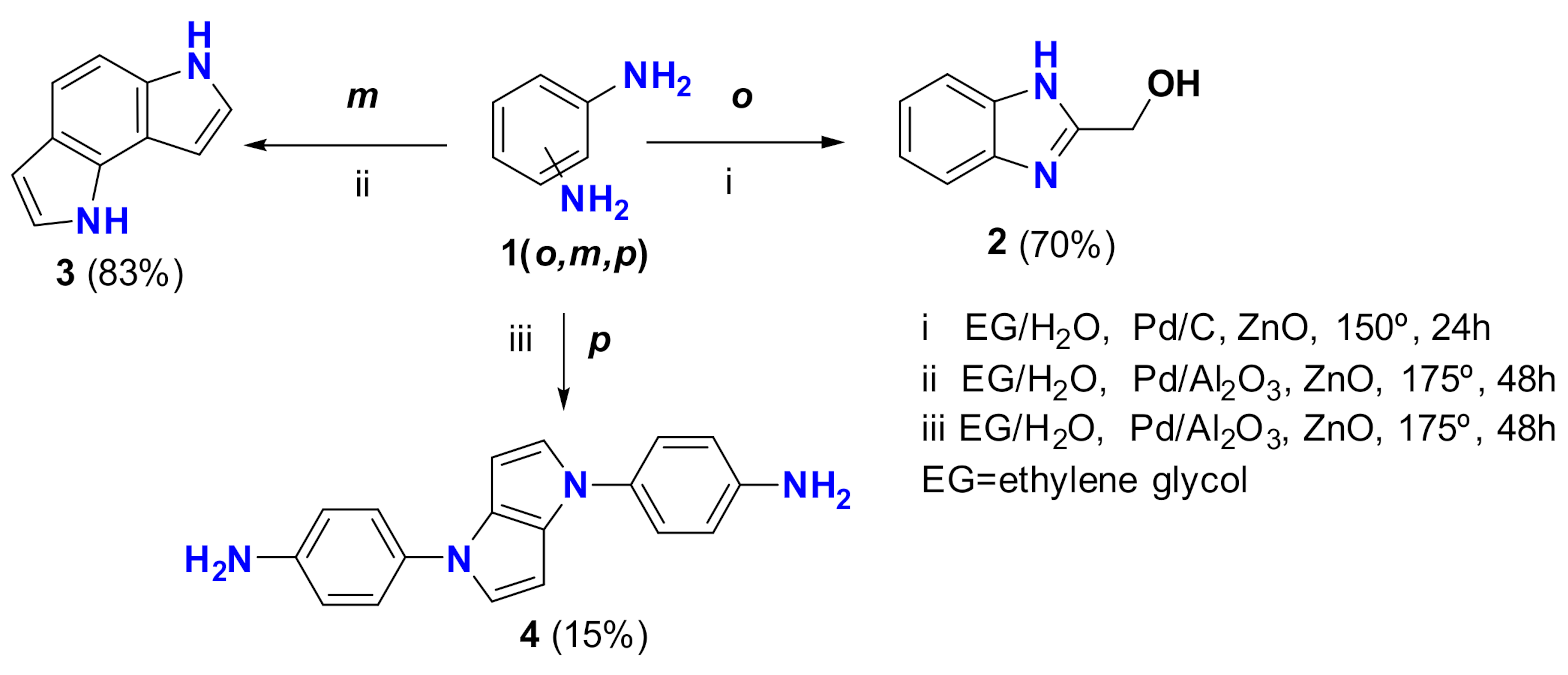
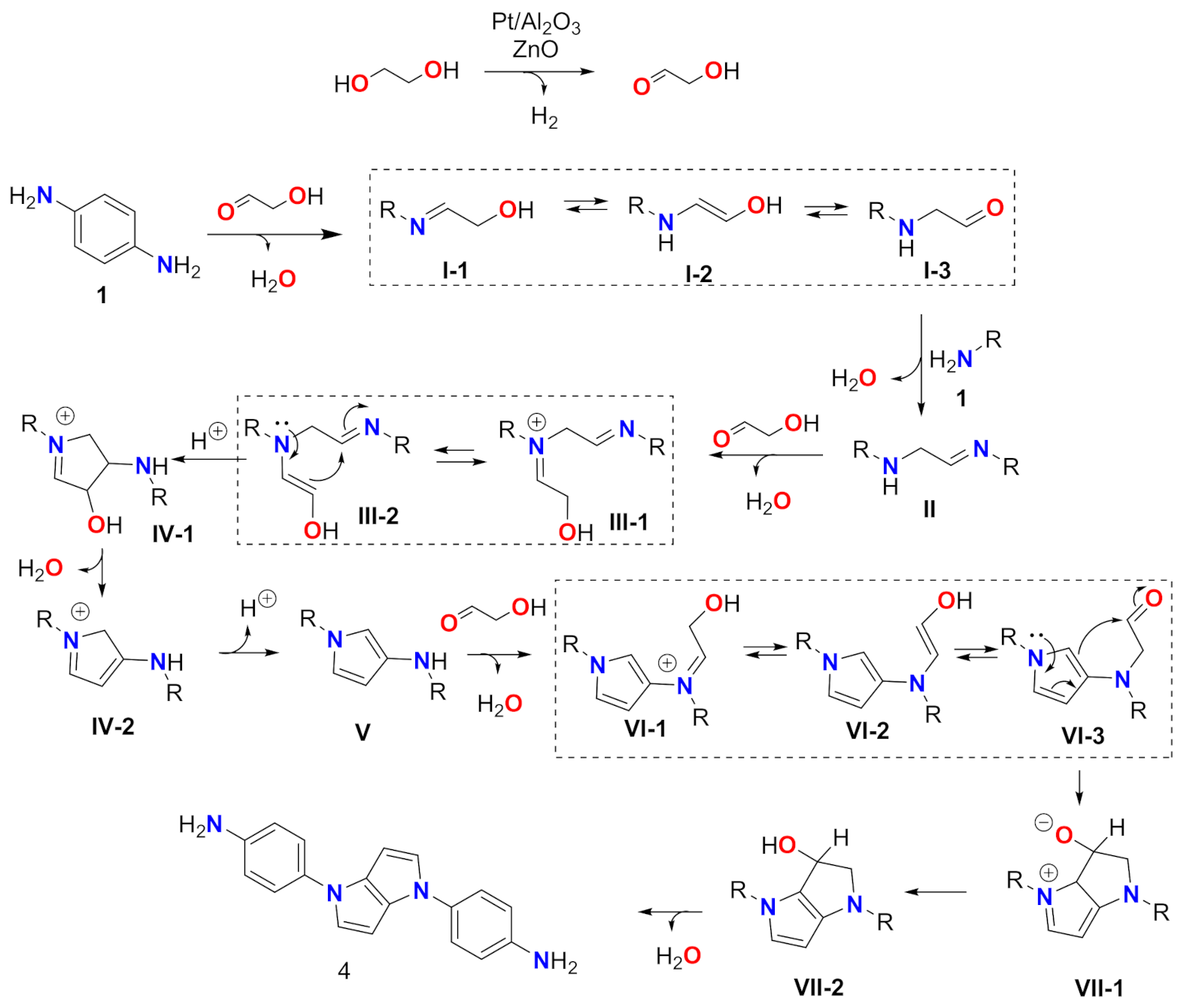
2. Results
3. Materials and Methods
Synthesis of 4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline (4)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Llabres-Campaner, P.J.; Ballesteros-Garrido, R.; Ballesteros, R.; Abarca, B. β-Amino alcohols from anilines and ethylene glycol through heterogeneous Borrowing Hydrogen reaction. Tetrahedron 2017, 73, 5552–5561. [Google Scholar] [CrossRef]
- Llabres-campaner, P.J.; Ballesteros-Garrido, R.; Ballesteros, R.; Abarca, B. Straight Access to Indoles from Anilines and Ethylene Glycol by Heterogeneous Acceptorless Dehydrogenative Condensation. J. Org. Chem. 2018, 83, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Williams, J.M.J. The give and take of alcohol activation. Science 2010, 329, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.H.S.A.; Slatford, P.A.; Williams, J.M.J. Borrowing hydrogen in the activation of alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Ramón, D.J.; Yus, M. Hydrogen autotransfer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef] [PubMed]
- Hille, T.; Irrgang, T.; Kempe, R. Synthesis of meta-Functionalized Pyridines by Selective Dehydrogenative Heterocondensation of β-and γ-Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Kallmeier, F.; Dudziec, B.; Irrgang, T.; Kempe, R. Manganese-Catalyzed Sustainable Synthesis of Pyrroles from Alcohols and Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 7261–7265. [Google Scholar] [CrossRef] [PubMed]
- Tasior, M.; Vakuliuk, O.; Koga, D.; Koszarna, B.; Górski, K.; Grzybowski, M.; Kielesiński, Ł.; Krzeszewski, M.; Gryko, D.T. The Method for the Large Scale Synthesis of Multifunctional 1,4-Dihydro-pyrrolo[3,2-b]pyrroles. J. Org. Chem. 2020, 85, 13529–13543. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, L.; Ballesteros, R.; Ballesteros-Garrido, R. 4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline. Molbank 2020, 2020, M1169. https://doi.org/10.3390/M1169
Giordano L, Ballesteros R, Ballesteros-Garrido R. 4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline. Molbank. 2020; 2020(4):M1169. https://doi.org/10.3390/M1169
Chicago/Turabian StyleGiordano, Lorenza, Rafael Ballesteros, and Rafael Ballesteros-Garrido. 2020. "4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline" Molbank 2020, no. 4: M1169. https://doi.org/10.3390/M1169
APA StyleGiordano, L., Ballesteros, R., & Ballesteros-Garrido, R. (2020). 4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline. Molbank, 2020(4), M1169. https://doi.org/10.3390/M1169



