Abstract
Afatinib is a 4-anilinoquinazoline tyrosine kinase inhibitor (TKI) in the form of a dimaleate salt which is indicated for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC). The most scalable route for the synthesis of this drug was reported in two Boehringer Ingelheim patents, in which the title compound, 4,7-dichloro-6-nitroquinazoline (IV), is an important intermediate. Compound IV is also present in a number of synthetic pathways for various 4,7-disubstituted quinazoline derivatives displaying high therapeutic potential. However, no detailed characterization of this popular compound has been reported, possibly due to its high instability. In this paper, IV was prepared in an overall yield of 56.1% by a 3-step process (condensation, nitration, and chlorination) from 2-amino-4-chlorobenzoic acid (I). The target compound has been for the first time fully characterized by melting point, mass-spectrometry, FT-IR, 1H-NMR and 13C-NMR spectroscopies.
1. Introduction
Quinazoline and quinazolinones scaffolds are present in a diverse range of biologically active compounds with huge therapeutic potential, including anticancer, antimicrobial, antiviral, antituberculosis, antifungal, antimalarial, anti-inflammatory, analgesic, and antidiabetic properties [1,2,3,4]. Many quinazoline derivatives, in particular the 4-anilinoquinazolines such as gefitinib, erlotinib, lapatinib, vandetanib, icotinib, afatinib and dacomitinib are approved as tyrosine kinase inhibitors (TKI) for the treatment of different cancers in targeted therapies [5,6]. Among those, afatinib dimaleate is a powerful second-generation TKI, irreversibly binding to both EGFR (epidermal growth factor receptor) and HER2 (human epidermal growth factor receptor 2). It is an approved anticancer drug marketed under the brand names Giotrif® (EU, Japan, Taiwan and Canada) and Gilotrif® (USA). It is indicated for patients with specific types EGFR mutation-positive non-small cell lung cancer [7,8].
The synthetic route to afatinib dimaleate can be derived from two Boehringer Ingelheim patents, comprising 10 reactions [9,10,11,12,13,14]. In that synthetic procedure, the compound 4,7-dichloro-6-nitroquinazoline (IV, CAS Registry number 162012-71-7) is a highly reactive intermediate. However, it was used in situ and thus no characterization was available. Similarly, in other synthetic routes of other bioactive quinazoline derivatives reported by SciFinder and Reaxys, compound IV was also synthesized and used directly in the next step without characterization [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], or only partially characterized by 1H-NMR [37]. The most detailed characterization of IV includes a melting point analysis, 1H-NMR and 13C-NMR spectroscopies, and an elemental analysis [38]. However, it appears that the reported NMR data for IV in this paper are close to those of the starting material (7-chloro-6-nitroquinazolin-4(3H)-one, III), indicating the possibility of a hydrolysis reaction which converts IV back to the starting material. In addition, the given elemental analysis did not present the percentage of each element, which offers no clarification over the identity of this compound. In this paper, compound IV has been for the first time fully characterized by melting point, mass-spectrometry, FT-IR, 1H-NMR and 13C-NMR spectroscopies.
2. Results and Discussion
The target compound IV was prepared in three steps from 2-amino-4-chlorobenzoic acid (I) in an overall yield of 56.1% (Scheme 1). The synthetic procedures were based on references [30,33,39] with some changes.
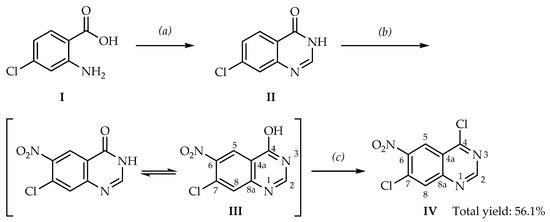
Scheme 1.
Synthesis of 4,7-dichloro-6-nitroquinazoline (IV). Reagents and conditions (yield): (a) HCO-NH2, reflux at 160 °C (82.3%); (b) HNO3/H2SO4 (84.7%); (c) SOCl2/DMF at 100 °C (91.3%).
Compound IV is an imidoyl halide (also known as imidyl or iminochlorides), a group of highly reactive organic compounds widely used as synthetic tools to produce a variety of compounds. It is often not necessary to isolate imidoyl halides because in situ generation and subsequent reactions can afford the desired derivatives in high yields [40,41,42]. In fact, our experimental observations demonstrate that IV is extremely sensitive to moisture and can be readily hydrolyzed giving back the starting material III. This phenomenon could explain the reason why the NMR-data in the aforementioned article [38] are so close to those of the starting material III. These data are presented in Table 1 and Table 2.

Table 1.
Reported and our data for 1H-NMR spectroscopies of III and IV.

Table 2.
Reported and our data for 13C-NMR spectroscopies of III and IV.
The data in Table 1 indicate that III is capable of tautomerism between the lactim (-C(OH)=N-) and lactam (-C(=O)-NH-) forms, displaying a huge difference in chemical shifts between the -OH and the -NH groups (12.79 ppm vs. 3.30 ppm). In the latter form, the -NH- signal is actually overlapped with the water signal if DMSO-d6 is used and may not be seen in a proton spectrum. As the reported proton chemical shifts for compound IV in [38] are similar to those of compound III in [30], we suspect that they are of the same chemical compound (Table 1). Indeed, the 1H-NMR values for compound IV in [38] do not match up with those of either our data or another paper [37], displaying large chemical shifts’ differences of 0.96 ppm, 0.44 ppm, and 0.43 ppm, respectively, for the aromatic hydrogens in DMSO-d6 [37,38]. We would not expect such large discrepancies in chemical shifts for the same compound using the same NMR solvent (DMSO-d6). When comparing our 1H-NMR data with those of [37], the differences are −0.38 ppm, 0.05 ppm, and 0.02 ppm, respectively, which indicate a high level of spectral similarities. These slight deviations could potentially originate from the fact that two different NMR solvents (DMSO-d6 vs. CDCl3) were used [44]. On the other hand, when examining the 13C-NMR data, we found strikingly similar values between IV of reference [38] and the starting material III (∆δ = 0–0.4 ppm, Table 2). These reported values are different from our 13C-NMR data of IV (∆δ = 0.4–5.4 ppm, Table 2). Altogether, both the 1H-NMR and 13C-NMR data combined indicate that the reported NMR data in [38] could potentially be that of the starting material III, and not the desired compound IV. One possible explanation could be the hydrolysis of the highly reactive compound IV back into the starting material III, a phenomenon we have observed in our laboratory.
Another interesting fact is that, when measuring mass spectrometry, we not only detected the peak of compound IV, but also its methoxy form (compound V), which possibly occurs due to alcoholysis by methanol (the solvent in MS). We show this in Scheme 2.
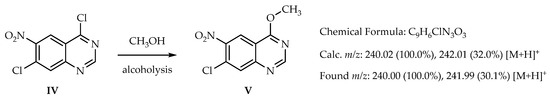
Scheme 2.
The formation of 7-chloro-4-methoxy-6-nitroquinazoline (V) by alcoholysis with methanol in MS.
On the whole, in the synthesis of IV, our control of anhydrous reaction conditions and the appropriate work-up procedures have made it feasible to synthesize and characterize this highly reactive compound for the first time. These data may be useful for further investigations in the synthesis process improvement of afatinib, its analogs and other biologically active quinazoline based compounds. All the mass, FT-IR, 1H-NMR and 13C-NMR spectra are presented in the Supplementary Material File.
3. Materials and Methods
3.1. General Information
The 2-amino-4-chlorobenzoic acid was purchased from Energy Chemical (Zhejiang, China) and used as received. Formamide (99.5%) was purchased from Scharlau Chemie (Barcelona, Spain). Thionyl chloride (99.5%) was purchased from Merck Schuchardt (Hohenbrunn, Germany). Dichloromethane (DCM, 99.5%), sufuric acid (98%) and N,N-dimethylformamide (DMF, 99.5%) was purchased from Xilong Scientific Co., Ltd. (Shantou, China). Fuming nitric acid (d 1.50 g/mL) was prepared by reaction of solid sodium nitrate and liquid sulfuric acid (98%), following distillation at b.p 82 °C.
The melting point was determined using a SRS EZ-Melt apparatus (Stanford Research Systems, Sunnyvale, CA, USA) and is uncorrected. MS was performed at a EVOQ Qube™ (Bruker, Billerica, MA, USA) or an LTQ Orbitrap XL™ (Thermo Scientific, Waltham, MA, USA) system. FT-IR spectra were recorded by a Perkin Elmer (Waltham, MA, USA) or Shimadzu (Kyoto, Japan) spectrometer. 1H- and 13C-NMR spectra were acquired with a 500 MHz Ascent spectrometer (Bruker, Billerica, MA, USA) using acetone-d6, DMSO-d6, or CDCl3 as the solvent. The reaction mixtures were monitored, and the purity of all products was checked by thin-layer chromatography (TLC) on silica gel 60 F254 plates (Merck, Darmstadt, Germany).
3.2. Synthetic Procedure
3.2.1. Preparation of 7-Chloroquinazolin-4(3H)-one (II)
The synthetic procedure for compound II was based on the method described in [39] with some modifications. A mixture of 2-amino-4-chlorobenzoic acid (I, 34.30 g, 0.20 mol, 1 equiv.), formamide (125.0 mL, 2.77 mol, 14 equiv.) was aerated with nitrogen, then stirred and refluxed for 1.5 h at 160 °C (the reaction was monitored by TLC with a 9:1 DCM/methanol mixture as eluent). The reaction mixture was cooled to 80 °C, then 500 mL of water was added. The mixture was cooled to −5 °C in 1 h and filtered. The precipitate was washed with water and dried at 60 °C to afford 7-chloroquinazolin-4(3H)-one (II) as a light brown solid (29.71 g, 82.3%), which was used for the next step without further purification. M.p 251.0–253.0 °C. Rf 0.60 (DCM/methanol, 9:1). MS (ESI+, MeOH), m/z: found 181.2 and 183.2 [M + H]+, C8H5ON2Cl requires [M + H]+ 181.0 and 183.0. FT-IR (KBr), νmax (cm−1): 3031 (C-H); 2964, 2919 (N-H); 1714 (C=O); 1693 (C=N); 1655, 1606 (C=C). 1H-NMR (acetone-d6), δ (ppm): 11.82 (br.s, 1H, H-3, NH); 8.17 (d, J = 8,5 Hz, 1H, H-5); 8.09 (s, 1H, H-2); 7.67 (d, J = 2.0 Hz, 1H, H-8); 7.51 (dd, J1 = 8.5 Hz, J2 = 2.0 Hz, 1H, H-6). 13C-NMR (acetone-d6), δ (ppm): 160.6 (C-4); 151.2 (C-8a); 147.3 (C-2); 140.2 (C-7); 128.8 (C-5); 127.7 (C-6); 127.6 (C-8); 122.8 (C-4a).
3.2.2. Preparation of 7-Chloro-6-nitroquinazolin-4(3H)-one (7-Chloro-4-hydroxy-6-nitroquinazoline, III)
The synthetic procedure for compound III was based on [30] with some modifications. Compound II (10.83 g, 0.060 mol) and sulfuric acid (120 mL) were added into a 500 mL two-neck round bottom flask. The mixture was cooled to 0 °C on ice and stirred until dissolution. Fuming nitric acid (120 mL) was slowly added to the mixture at 0 °C, and the mixture was stirred for 1.5 h at 30 °C. After completion of reaction as indicated by TLC, 10% NaOH solution was slowly added to the reaction mixture until a precipitate was formed (pH ~7). The mixture was then filtered to furnish a light yellow solid (III). The compound was purified by redissolving in HCl (5M) and then neutralizing this solution with NaOH (10%) to pH 6–7 to furnish the pure precipitate which was then filtered, washed with water and dried at 60 °C to furnish 7-chloro-6-nitroquinazolin-4(3H)-one (III) as a light yellow solid (11.46 g, 84.7%). M.p 263.5–265.0 °C. Rf 0.34 (DCM/methanol, 20:1). MS (ESI+, MeOH), m/z: found 225.9 [M + H]+, C8H4O3N3Cl requires [M + H]+ 225.9. FT-IR (KBr), νmax (cm−1): 3452, 3215 (O-H); 3091, 3012 (C-H); 1696 (C=O); 1666, 1612 (C=N); 1523 (C=C); 1336 (NO2). 1H-NMR (DMSO-d6), δ (ppm): 12.73 (br.s, 1H, OH); 8.64 (s, 1H, H-5); 8.27 (s, 1H, H-2); 7.97 (s, 1H, H-8). 13C-NMR (DMSO-d6), δ (ppm): 159.3 (C-4); 151.5 (C-8a); 149.6 (C-2); 144.7 (C-6); 130.4 (C-7); 129.9 (C-5); 124.2 (C-8); 121.7 (C-4a).
3.2.3. Preparation of 4,7-Dichloro-6-nitroquinazoline (IV)
The synthetic procedure for compound IV was based on [33] with some modifications. A mixture of compound III (6.09 g, 0.027 mol), thionyl chloride (48.0 mL, 0.661 mol) and N,N-dimethyl-formamide (0.25 mL) was melted and stirred at 100 °C for 2 h. The reaction mixture was allowed to cool down and excess thionyl chloride was removed by rotary evaporation under reduced pressure. Toluene (40 mL) was added to the residue and the mixture was evaporated again to completely remove volatile matter. The precipitate was washed with diethyl ether and dried in the desiccator to obtain 4,7-dichloro-6-nitroquinazoline (IV) as a yellow solid (6.02 g, 91.3%). M.p: 269.0–270.5 °C. Rf: 0.87 (DCM/methanol, 20:1). MS (ESI+, MeOH), m/z: found 244.4 [M + H]+, 240.00 and 241.99 [methoxy form]; C8H3O2N3Cl2 requires [M + H]+ 244.0 and 246.0. FT-IR (KBr), νmax (cm−1): 3089 (C-H); 1726, 1645, 1610 (C=N); 1546 (C=C); 1527, 1323 (NO2). 1H-NMR (CDCl3), δ (ppm): 9.18 (s, 1H, H-2); 8.76 (s, 1H, H-5); 8.30 (s, 1H, H-8). 13C-NMR (CDCl3), δ (ppm): 163.6 (C-4); 156.9 (C-2); 151.6 (C-8a); 147.5 (C-6); 132.8 (C-7); 132.2 (C-8); 123.5 (C-5); 122.1 (C-4a).
Supplementary Materials
Spectral data of starting material I, intermediates II, III and title compound IV are available online, Figure S1: FT-IR spectrum of compound 2-amino-4-chlorobenzoic acid (I), Figure S2: 1H-NMR spectrum of compound 2-amino-4-chlorobenzoic acid (I), Figure S3: 13C-NMR spectrum of compound 2-amino-4-chlorobenzoic acid (I), Figure S4: MS spectrum of compound 7-chloroquinazolin-4(3H)-one (II), Figure S5: FT-IR spectrum of compound 7-chloroquinazolin-4(3H)-one (II), Figure S6: 1H-NMR spectrum of compound 7-chloroquinazolin-4(3H)-one (II), Figure S7: 13C-NMR spectrum of compound 7-chloroquinazolin-4(3H)-one (II), Figure S8: MS spectrum of compound 7-chloro-6-nitroquinazolin-4(3H)-one (III), Figure S9: FT-IR spectrum of compound 7-chloro-6-nitroquinazolin-4(3H)-one (III), Figure S10: 1H-NMR spectrum of compound 7-chloro-6-nitroquinazolin-4(3H)-one (III), Figure S11: 13C-NMR spectrum of compound 7-chloro-6-nitroquinazolin-4(3H)-one (III), Figure S12: MS spectrum of compound 4,7-dichloro-6-nitroquinazoline (IV), Figure S13: FT-IR spectrum of compound 4,7-dichloro-6-nitroquinazoline (IV), Figure S14: 1H-NMR spectrum of compound 4,7-dichloro-6-nitroquinazoline (IV), Figure S15: 13C-NMR spectrum of compound 4,7-dichloro-6-nitroquinazoline (IV).
Author Contributions
T.N.N. and T.H.T. synthesized the compounds. T.N.N. wrote the manuscript. N.S.H.D., V.G.N. and D.L.N. designed the experiments. V.H.N. analyzed spectroscopic data. V.H.N. and N.T.T. edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Hanoi University of Pharmacy and Thainguyen University of Medicine and Pharmacy for financial support and research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Iqbal, J.; Saeed, A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Hong, J. Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur. J. Med. Chem. 2019, 170, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Shagufta; Ahmad, I. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. MedChemComm 2017, 8, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, C.; Iaccarino, S.; Dolce, P.; Pagliuca, M.; Izzo, M.; Scafuri, L.; Costabile, F.; Riccio, V.; Ribera, D.; Mucci, B.; et al. Predictors of outcomes in patients with EGFR-mutated non-small cell lung cancer receiving EGFR tyrosine kinase inhibitors: A systematic review and meta-analysis. Cancers 2019, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D.; Keating, G.M. Afatinib in advanced NSCLC: A profile of its use. Drugs Ther. Perspect. 2018, 34, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Boehringer Ingelheim International GmbH. Available online: https://www.boehringer-ingelheim.com/oncology/lung-cancer/giotrif-gilotrif-afatinib-approved-nsclc (accessed on 4 February 2020).
- Himmelsbach, F.; Langkopf, E.; Blech, S.; Jung, B.; Baum, A.; Solca, F. Quinazoline Derivatives, Medicaments Containing Said Compounds, Their Utilization and Method for the Production Thereof. PCT Patent WO200250043A1, 27 June 2002. [Google Scholar]
- Soyka, R.; Rall, W.; Schnaubelt, J.; Sieger, P.; Kulinna, C. Process for Preparing Amino Crotonyl Compounds. U.S. Patent 20050085495A1, 21 April 2005. [Google Scholar]
- Schroeder, J.; Dziewas, G.; Fachinger, T.; Jaeger, B.; Reichel, C.; Renner, S. Process for Preparing Aminocrotonylamino-Substituted Quinazoline Derivatives. U.S. Patent US8188274B2, 29 May 2012. [Google Scholar]
- Xu, X. Method for Preparing Afatinib and Intermediate Thereof. PCT Patent WO2014180271A1, 13 November 2014. [Google Scholar]
- Xu, X. Afatinib and Preparation Method of Intermediate Thereof. PCT Patent WO2014183560A1, 20 November 2014. [Google Scholar]
- Ding, H.X.; Leverett, C.A.; Kyne, R.E., Jr.; Liu, K.K.C.; Fink, S.J.; Flick, A.C.; O’Donnell, C.J. Synthetic approaches to the 2013 new drugs. Bioorg. Med. Chem. 2015, 23, 1895–1922. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.J. Quinazoline Derivatives and Their Use as Anti-Cancer Agents. European Patent EP0635498A1, 25 January 1995. [Google Scholar]
- Barker, A.J. Quinazoline Derivative. PCT Patent WO1996033981A1, 17 May 1996. [Google Scholar]
- Brown, D.S.; Morris, J.J.; Thomas, A.P. Aniline Derivatives. PCT Patent WO1996015118A1, 23 May 1996. [Google Scholar]
- Barker, A.J. Quinazoline Derivatives. PCT Patent WO9616960A1, 6 June 1996. [Google Scholar]
- Thomas, A.P.; Hennequin, L.F.A.; Johnstone, C.; Stokes, E.S.E.; Lohmann, J.-J.M.; Clayton, E. Quinazoline Derivatives and Pharmaceutical Compositions Containing Them. PCT Patent WO1998013354A1, 2 April 1998. [Google Scholar]
- Barker, A.J. Quinazoline Derivative. U.S. Patent US5952333A, 14 September 1999. [Google Scholar]
- Tobe, M.; Isobe, Y.; Tomizawa, H.; Matsumoto, M.; Obara, F.; Nagasaki, T.; Hayashi, H. Structure-activity relationships of quinazoline derivatives: Dual-acting compounds with inhibitory activities toward both TNF-α production and T cell proliferation. Bioorg. Med. Chem. Lett. 2001, 11, 545–548. [Google Scholar] [CrossRef]
- Tobe, M.; Isobe, Y.; Tomizawa, H.; Nagasaki, T.; Takahashi, H.; Fukazawa, T.; Hayashi, H. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-κB activation. Bioorg. Med. Chem. 2003, 11, 383–391. [Google Scholar] [CrossRef]
- Matsuno, K.; Seishi, T.; Nakajima, T.; Ichimura, M.; Giese, N.A.; Yu, J.-C.; Oda, S.; Nomoto, Y. Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. Part 4: Structure-activity relationships for substituents on the quinazoline moiety of 4-[4-(N-substituted(thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 3001–3004. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Lee, S.; Choi, N.S.; Lee, J.K.; Moon, S.K.; Choi, H.; Kim, S.J.; Kim, Y.H.; Kang, S.K.; Lee, H.W.; et al. Quinazoline Derivative as Phosphodiesterase Inhibitor and a Process for Preparing the Same. PCT Patent WO2008020711A1, 21 February 2008. [Google Scholar]
- Guo, J.; Wang, M.; Jiang, Y.; Zhang, X. Quinazoline Derivatives, Preparation Methods and Uses Thereof. European Patent EP1990337A1, 12 November 2008. [Google Scholar]
- Jiang, Y.; Guo, J. Medical Application of 4-Anilinoquinazoline Derivatives. CN Patent CN101347433A, 21 January 2009. [Google Scholar]
- Tang, T.; Yu, L.; Feng, H.; Yan, Q.; Wang, B.; Wang, Z.; Zhu, D.; Chen, H. Heterocyclic Compound of 4-Aminoquinazoline Useful in Treatment of Cancer and Its Application. CN Patent CN102942561A, 27 February 2013. [Google Scholar]
- Zhang, K.; Cao, D.; Xue, N.; Shi, Q.; Du, Y.; Dong, M. Phenylurea-Quinazoline Coupling Compounds: Preparation Method, Medicine Composition and Medicinal Use. CN Patent CN103382182A, 6 November 2013. [Google Scholar]
- Zhang, X.; Peng, T.; Ji, X.; Li, J.; Tong, L.; Li, Z.; Yang, W.; Xu, Y.; Li, M.; Ding, J.; et al. Design, synthesis and biological evaluation of novel 4-anilinoquinazolines with C-6 urea-linked side chains as inhibitors of the epidermal growth factor receptor. Bioorg. Med. Chem. 2013, 21, 7988–7998. [Google Scholar] [CrossRef] [PubMed]
- Buha, V.M.; Rana, D.N.; Chhabria, M.T.; Chikhalia, K.H.; Mahajan, B.M.; Brahmkshatriya, P.S.; Shah, N.K. Synthesis, biological evaluation and QSAR study of a series of substituted quinazolines as antimicrobial agents. Med. Chem. Res. 2013, 22, 4096–4109. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Li, X.; Zhang, X.; Liu, Y.; Gong, N.; Zhou, Y.; Chen, K. Artemisinin Derivatives: Preparation Process and Application. PCT Patent WO2014023081A1, 13 February 2014. [Google Scholar]
- Tang, T.; Yu, L.; Feng, H.; Yan, Q.; Wang, B.; Wang, Z.; Zhu, D.; Chen, H. 4-Quinazolinamine Heterocyclic Compound and Use Thereof. PCT Patent WO2014071824A1, 15 May 2014. [Google Scholar]
- Yuan, J.; Han, N.; Yi, H.; Wang, Y.; Yang, S.; Wong, J.C. Potent Small Molecule Inhibitors of Autophagy, and Methods of Use Thereof. PCT Patent WO2014145512A3, 31 December 2014. [Google Scholar]
- Lai, Y.; Pang, J.; Luo, M.; Chen, F.; Wang, P.; Zhang, Y. Preparation of α-Cyano-α,β-Unsaturated Amides as Antitumor Agents. CN Patent CN104774184A, 15 July 2015. [Google Scholar]
- Zhang, K.; Cao, D.; Xue, N.; Shi, X.; Lu, K.; Gu, J.; Wang, L. Aryl Formyl Urea-Coupled Quinazoline Compound Useful in Treatment of Cancer and Its Preparation. CN Patent CN105753793A, 13 July 2016. [Google Scholar]
- Min, J.; Guo, K.; Suryadevara, P.K.; Zhu, F.; Holbrook, G.; Chen, Y.; Feau, C.; Young, B.M.; Lemoff, A.; Connelly, M.C.; et al. Optimization of a novel series of ataxia-telangiectasia mutated kinase inhibitors as potential radiosensitizing agents. J. Med. Chem. 2016, 59, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tu, Z.; Hao, H.; Yao, Y.; Qiu, Y.; Yao, H.; Qiang, L.; Chen, D. A Kind of Benzo-Aza Virtue Cyclics and Its Preparation Method and Application. CN Patent CN107674059A, 9 February 2018. [Google Scholar]
- Bridges, A.J.; Zhou, H.; Cody, D.R.; Rewcastle, G.W.; McMichael, A.; Showalter, H.D.H.; Fry, D.W.; Kraker, A.J.; Denny, W.A. Tyrosine kinase inhibitors. 8. An unusually steep structure−activity relationship for analogues of 4-(3-bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a potent inhibitor of the epidermal growth factor receptor. J. Med. Chem. 1996, 39, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.T.; Wang, G.F.; Yang, Y.Q.; Jin, F.; Wang, Y.; Xie, X.Y.; Mach, R.H.; Huang, Y.S. Synthesis and pharmacological evaluation of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline derivatives as sigma-2 receptor ligands. Eur. J. Med. Chem. 2018, 147, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, NY, USA, 1968; pp. 55–56. [Google Scholar]
- Li, H.G.; Kim, C.K.; Lee, B.S.; Kim, C.K.; Rhee, S.K.; Lee, I. Nucleophilic substitution at the imidoyl carbon atom: Intermediate mechanistic and reactivity behavior between carbonyl and vinyl carbon substitution. J. Am. Chem. Soc. 2001, 123, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.J.; Bilodeau, M.T. A mild method for the formation and in situ reaction of imidoyl chlorides: Conversion of pyridine-1-oxides to 2-aminopyridine amides. Org. Lett. 2002, 4, 3127–3129. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Wang, W.; Xiao, Y. Preparation Method for Afatinib Intermediate 6-Nitro-7-Chloro-4-Quinazolinone. CN Patent CN105712940A, 29 June 2016. [Google Scholar]
- Abraham, R.J.; Byrne, J.J.; Griffiths, L.; Perez, M. 1H chemical shifts in NMR: Part 23, the effect of dimethyl sulphoxide versus chloroform solvent on 1H chemical shifts. Magn. Reson. Chem. 2006, 44, 491–509. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).