Abstract
The X-ray structure of the title compound has been determined and the structure shows an exo-configured planar dithiolanone ring. This is in contrast to the few previous dithiolanones to be characterised crystallographically, which are all twisted.
1. Introduction
The title compound 1 was first mentioned in 1985 [1], when it was formed by mercuric acetate oxidation of the corresponding 1,3-dithiolane-2-thione 2 (Scheme 1). However, in that report it was treated as a synthetic intermediate and no analytical or spectroscopic data were given. A short time later, a second preparative method was described [2] involving reaction of the 1,2,3-trithiolane 3, formed from norbornene 4 and sulfur [3], with dichlorocarbene under phase-transfer conditions. In this report it was suggested that the trithiolane 3 reacts with dichlorocarbene to give the thione, 2, but the mechanism by which 2 was converted into 1 was unclear. Perhaps the simplest preparation of 1 involves direct treatment of norbornene 4 with diisopropyl xanthogen disulfide and the radical initiator azobis(isobuytronitrile) (AIBN), which affords 1 directly in 74% yield [4]. Although compound 1 has been characterised by melting point, 1H and 13C-NMR and IR spectroscopy, and elemental analysis [2], its X-ray structure has not been investigated. We have obtained compound 1 repeatedly in low yield as a by-product arising from oxidative degradation of the adduct, 5, formed from norbornene 4 and Bu3P × CS2. The ylene 5 can be exploited synthetically in a Wittig reaction with aldehydes to give 2-alkylidene-1,3-dithiolanes [5,6,7] or, in the presence of extra CS2, in a 1,3-dipolar cycloaddition process with dipolarophiles such as dimethyl acetylenedicarboxylate (DMAD) to give dihydrotetrathiafulvalenes [8]. Furthermore, it can also undergo hydrolysis to give the corresponding 1,3-dithiolane [9]. We describe here determination of the molecular and crystal structure of 1 by X-ray diffraction. The structure is compared with the few crystal structures previously reported for 1,3-dithiolan-2-ones.
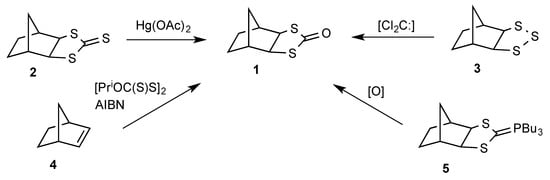
Scheme 1.
Synthetic routes to 1.
2. Results
A sample of compound 1 suitable for X-ray diffraction was obtained from chromatographic purification of the product obtained by reaction of 5 with propargylaldehyde, HC≡C–CHO, and CS2 (<5% yield). The resulting molecular structure is shown in Figure 1 and selected bond lengths and angles are given in Table 1. The structure features an exo-configured dithiolanone ring, which is essentially planar with the expected long C-S bond lengths and correspondingly smaller internal angles at sulfur. The crystal structure features a centrosymmetric arrangement of four molecules in the unit cell (Figure 2). Rather surprisingly, a search of the Cambridge Structural Database (CSD, March 2020 update) revealed only five previous structures containing the 1,3-dithiolan-2-one fragment with at least one sp3 carbon in the ring (Figure 3). These are the parent compound 6 [10], the polychlorinated compound 7 in which the dithiolanone ring is tetrasubstituted [11], the bicyclic diester 8 with a trans-disubstituted dithiolanone ring [12], and the Diels–Alder dimer of 4,5-bis(methylene)-1,3-dithiolan-2-one 9 and the derived cobalt complex 10 [13], both of which have the dithiolanone ring fully substituted but with one spiro sp3 centre and one sp2 centre.
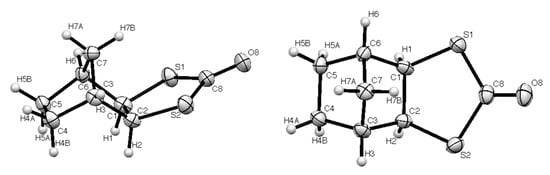
Figure 1.
Two views of the molecular structure of 1 with numbering scheme (ORTEP probability ellipsoids at 50%).

Table 1.
Selected bond lengths and angles.
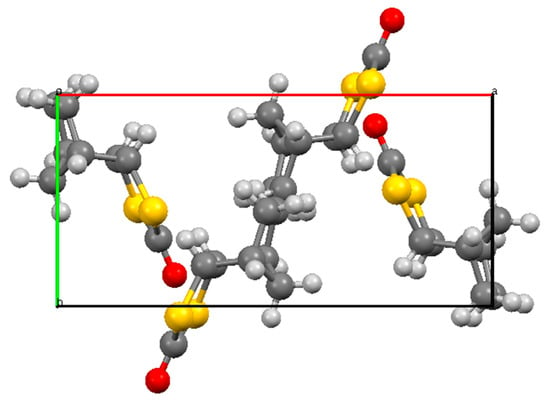
Figure 2.
Unit cell of 1 viewed along the c-axis showing the four molecules present.
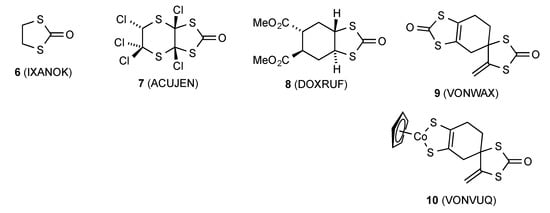
Figure 3.
A survey of crystallographically characterised 1,3-dithiolan-2-ones with Cambridge Structural Database (CSD) reference codes.
While comparison of the internal angles at sulfur and at C=O show all six compounds to have rather similar values, the key difference observed between the molecular structure of 1 and the previous structures 6–10 is the degree of planarity in the dithiolanone ring. This is readily quantified by examining the torsion angle S-C-C-S for the six compounds (Table 2). While the carbonyl C(2) carbon is completely planar in all cases, it is only in the case of 1 where the whole dithiolanone ring is fused cis to the rigid bicyclo[2.2.1] skeleton that all five ring atoms are essentially coplanar, corresponding to a S-C-C-S torsion angle near to zero. In all the other cases this torsion angle is significantly greater, corresponding to a twisting of the ring, and it appears that even in the absence of substituent effects in 6 this twisted conformation is preferred.

Table 2.
Key angles in the structures of dithiolanones (°).
In summary, the X-ray crystal structure of the norbornane-fused 1,3-dithiolan-2-one 1 shows it to adopt an exo configuration with a planar heterocyclic ring, in contrast to the twisted shape adopted by the small number of other 1,3-dithiolan-2-ones for which crystal structures are known.
3. Experimental
3,5-Dithiatricyclo[5.2.1.02,6]decan-4-one (1): Crystal data for C8H10OS2, M = 186.28 g mol−1, colourless prism, crystal dimensions 0.25 × 0.12 × 0.05 mm, orthorhombic, space group Pna21, a = 13.134(6), b = 6.336(3), c = 9.999(4) Å, V = 832.1(6) Å3, Z = 4, Dcalc = 1.487 g cm−3, T = 93 K, R1 = 0.0430, Rw2 = 0.1051 for 1350 reflections with I > 2σ(I), and 101 variables, Rint 0.0530, Goodness of fit on F2 1.122. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71075 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 1989785. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. The structure was solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL Version 2018/3 [14]). Hydrogen atoms were assigned riding isotropic displacement parameters and constrained to idealised geometries. The data did not allow determination of the polarity of the axis.
Author Contributions
N.S.K. prepared the compound; A.M.Z.S. collected the X-ray data and solved the structure; R.A.A. designed the experiments, analysed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Russell, G.A.; Law, W.C.; Zaleta, M. Aliphatic semidiones. 44. Spin probes derived from dithiols. J. Am. Chem. Soc. 1985, 107, 4175–4182. [Google Scholar] [CrossRef]
- Ghosh, T. Reaction of trithiolanes with dihalocarbenes under phase-transfer catalysis. A convenient synthesis of trithiocarbonates and thionocarbonates. J. Org. Chem. 1990, 55, 1146–1147. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Ghosh, T. Sulfuration of the norbornene double bond. J. Org. Chem. 1987, 52, 4937–4943. [Google Scholar] [CrossRef]
- Gareau, Y.; Beauchemin, A. Free Radical Reaction of Diisopropyl Xanthogen Disulfide with Unsaturated Systems. Heterocycles 1998, 48, 2003–2017. [Google Scholar] [CrossRef]
- Aitken, R.A.; Massil, T.; Raut, S.V. Cycloaddition of Bun3P·CS2: direct one-pot conversion of strained double bonds to 2-alkylidene-1,3-dithiolanes. J. Chem. Soc. Chem. Commun. 1994, 2603–2604. [Google Scholar] [CrossRef]
- Aitken, R.A.; Carcas, K.; Hill, L.; Massil, T.; Raut, S.V. Cycloaddition of Bun3P·CS2: Direct one-pot conversion of strained double bonds to 2-alkylidene-1,3-dithiolanes. Tetrahedron 1997, 53, 2261–2270. [Google Scholar] [CrossRef]
- Aitken, R.; Hill, L.; Massil, T.; Hursthouse, M.B.; Malik, K. Cycloaddition of Bu3P·CS2: Formation of extended bis- and tris-1,3-dithiolanes and dithiolane-containing polymers. Tetrahedron 1997, 53, 10441–10450. [Google Scholar] [CrossRef]
- Aitken, R.A.; Hill, L.; Lightfoot, P. Direct one pot construction of norbornane-fused dihydrotetrathiafulvalenes. Tetrahedron Lett. 1997, 38, 7927–7930. [Google Scholar] [CrossRef]
- Aitken, R.A.; Aitken, K.M.; Lambert, S.; Playfair, R.; Wilson, N.J. Synthesis of Norbornane-Fused 1,3-Dithiolanes and Evaluation of 1,3-Dithiolane-Containing Polymers as Absorbants for Mercury(II) Salts. Heterocycles 2012, 84, 1113–1122. [Google Scholar] [CrossRef]
- Reinheimer, E.; Bacsa, J.; Dunbar, K.R. 1,3-Dithiolan-2-one. Acta Crystallogr. Sect. E 2004, 60, o1206–o1207. [Google Scholar] [CrossRef]
- Dautel, O.J.; Fourmigué, M. Polyhalogenated BEDT-TTF through chlorination (SO2Cl2, Cl2) and fluorination (®Selectfluor, XeF2) of 5,6-dihydro[1,3]dithiolo[4,5-b][1,4]dithiin-2-one. J. Chem. Soc. Perkin Trans. 1 2001, 3399–3402. [Google Scholar] [CrossRef]
- Dotsenko, I.A. Convenient synthesis of 1,3-dithiolane-2-thiones: Cyclic trithiocarbonates as conformational locks. Arkivoc 2014, 16–41. [Google Scholar] [CrossRef]
- Masui, T.; Nomura, M.; Kobayashi, Y.; Terada, K.; Fujita-Takayama, C.; Sugiyama, T.; Kajitani, M. CpCoI-mediated Diels–Alder Reaction Forming Dimeric 1,3-Dithiol-2-one Derivative with Spiro Structure and Successive Formation of Novel Cobalt Dithiolene Complex. Chem. Lett. 2008, 37, 1032–1033. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).