4-(2-Bromovinyl)benzocyclobutene
Abstract
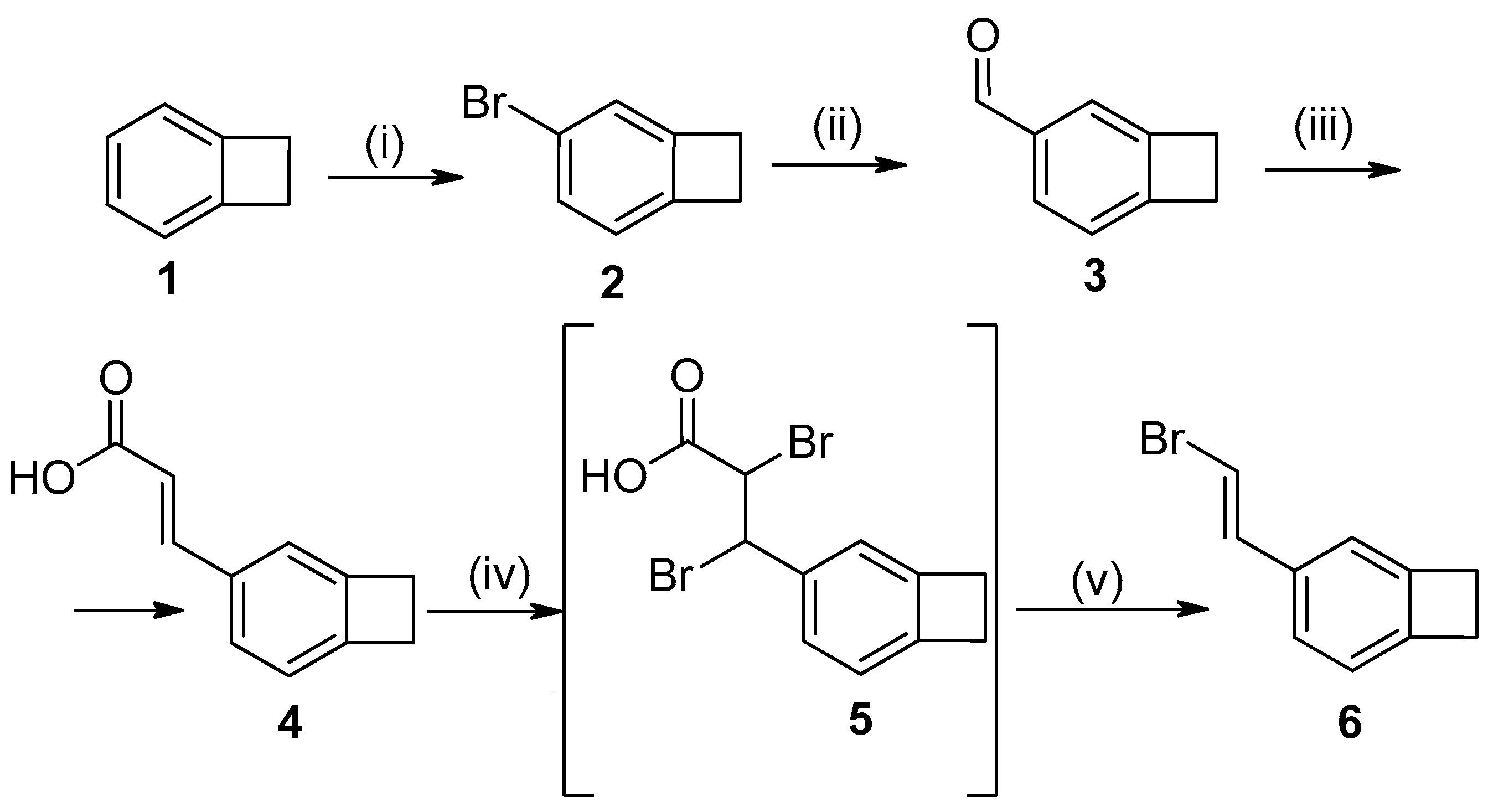
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. 4-Bromobenzocyclobutene (2)
3.2. Benzocyclobutene-4-carbaldehyde (3)
3.3. 4-(Bicyclo[4.2.0]octa-1,3,5-triene-3-yl)propene-2-oic acid (4)
3.4. 4-(2-Bromovinyl)benzocyclobutene (6)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, F.; Jin, K.; Wang, J.; Luo, Y.; Sun, J.; Fang, Q. New fluoropolymers having both low water uptake and a low dielectric constant. Macromol. Chem. Phys. 2015, 216, 2302–2308. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, J.; Zhao, X.; Yang, S.; Fan, L. Synthesis and characterization of siloxane resins derived from silphenylene-siloxane copolymers bearing benzocyclobutene pendant groups. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 7868–7881. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Sun, J.; Fang, Q. High performance low dielectric polysiloxanes with high thermostability and low water uptake. Mater. Chem. Front. 2018, 2, 1397–1402. [Google Scholar] [CrossRef]
- Que, X.; Yan, Y.; Qiu, Z. Synthesis and characterization of benzocyclobutene-terminated imides with high organosolubility. Eur. Polym. J. 2018, 103, 410–420. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Y.; Xiao, F. Synthesis, thermal and mechanical properties of benzocyclobutene-functionalized siloxane thermosets with different geometric structures. Eur. Polym. J. 2012, 48, 751–760. [Google Scholar] [CrossRef]
- Zuo, X.; Yu, R.; Shi, S.; Feng, Z.; Li, Z.; Yang, S.; Fan, L. Synthesis and characterization of photosensitive benzocyclobutene-functionalized siloxane thermosets. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 6246–6258. [Google Scholar] [CrossRef]
- Xie, L.; Yang, J.; Zhu, F.; Yang, H.; Liu, C. Synthesis and characterization of benzocyclobuten-4-yl acrylate monomer and its radical polymerization. Chin. J. Polym. Sci. 2010, 28, 877–885. [Google Scholar] [CrossRef]
- Yang, J.; Xie, L.; Zhu, F.; Sui, H.; Li, H.; Huang, Y. Incorporation of benzocyclobutene cross-linkable moieties in poly(methyl acrylate): A novel approach to shape-memory polymers accompanied with microphase separation. J. Macromol. Sci. B. 2011, 50, 2129–2139. [Google Scholar] [CrossRef]
- Hu, H.; Liu, L.; Li, Z.; Zhao, C.; Huang, Y.; Chang, G.; Yang, J. Benzocyclobutene/vinylphenyl-introduced polycarbosilanes with low dielectric constant, high temperature performance and photopatternability. Polymer 2015, 66, 58–66. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Hu, H.; Wei, X.; Yu, H.; Yang, J. Photoactive Polymers with Benzocyclobutene/Silacyclobutane Dual Crosslinked Structure and Low Dielectric Constant. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 1920–1928. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Hu, H.; Wei, X.; Yu, H.; Yang, J. Synthesis of poly(silmethylene)s via ring-opening polymerization of benzocyclobutene functionalized disilacyclobutene and their low-dielectric and thermal properties. Polym. Adv. Technol. 2017, 28, 1480–1488. [Google Scholar] [CrossRef]
- Cho, T.P.; Hejun, L.; Hao, Z.; Yiqian, C.; Hongbo, F.; Shenglan, W.; Li, W. Bicyclo-Substituted Pyrazolon Azo Derivatives, Preparation Process and Pharmaceutical Use Thereof. U.S. Patent EP2236500, 6 October 2010. [Google Scholar]
- Simpson, C.J.; Fitzhenry, M.J.; Stamford, N.P.J. Preparation of vinylphenols from 2- and 4-hydroxybenzaldehydes. Tetrahedron Lett. 2005, 46, 6893–6896. [Google Scholar] [CrossRef]
- Grovenstein, E., Jr.; Lee, D.E. The stereochemistry and mechanism of the transformation of cinnamic acid dibromide to β-bromostyrene. J. Am. Chem. Soc. 1953, 75, 2639–2644. [Google Scholar] [CrossRef]
- Roth, M.; Ahles, M.; Gawrisch, C.; Schwalm, T.; Schmechel, R.; Melzer, C.; von Seggern, H.; Rehahn, M. Rodlike Tetracene Derivatives. Chem. Eur. J. 2017, 23, 13445–13454. [Google Scholar] [CrossRef] [PubMed]
- Harth, E.; Van Horn, B.; Lee, V.Y.; Germack, D.S.; Gonzales, C.P.; Miller, R.D.; Hawker, C.J. A Facile Approach to Architecturally Defined Nanoparticles via Intramolecular Chain Collapse. J. Am. Chem. Soc. 2002, 124, 8653–8660. [Google Scholar] [CrossRef] [PubMed]
- Romer, D.; Yonkey, M.; Gallagher, M.; Wang, K.; Liu, X.; Thibault, R.; Ho, K.; Prokopowicz, G.; O’Connor, C.; Iagodkine, E.; et al. Arylcyclobutenes. U.S. Patent EP 3144359A1, 22 March 2017. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudov, K.A.; Levchenko, K.S.; Shmelin, P.S.; Grebennikov, E.P. 4-(2-Bromovinyl)benzocyclobutene. Molbank 2020, 2020, M1116. https://doi.org/10.3390/M1116
Chudov KA, Levchenko KS, Shmelin PS, Grebennikov EP. 4-(2-Bromovinyl)benzocyclobutene. Molbank. 2020; 2020(1):M1116. https://doi.org/10.3390/M1116
Chicago/Turabian StyleChudov, Konstantin A., Konstantin S. Levchenko, Pavel S. Shmelin, and Evgeny P. Grebennikov. 2020. "4-(2-Bromovinyl)benzocyclobutene" Molbank 2020, no. 1: M1116. https://doi.org/10.3390/M1116
APA StyleChudov, K. A., Levchenko, K. S., Shmelin, P. S., & Grebennikov, E. P. (2020). 4-(2-Bromovinyl)benzocyclobutene. Molbank, 2020(1), M1116. https://doi.org/10.3390/M1116




