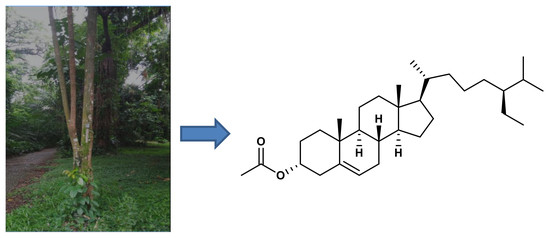
(22E,24S)-24-Propylcholest-5en-3α-acetate: A New Steroid from the Stembark Aglaia angustifolia (Miq.) (Meliaceae)
Abstract
1. Introduction
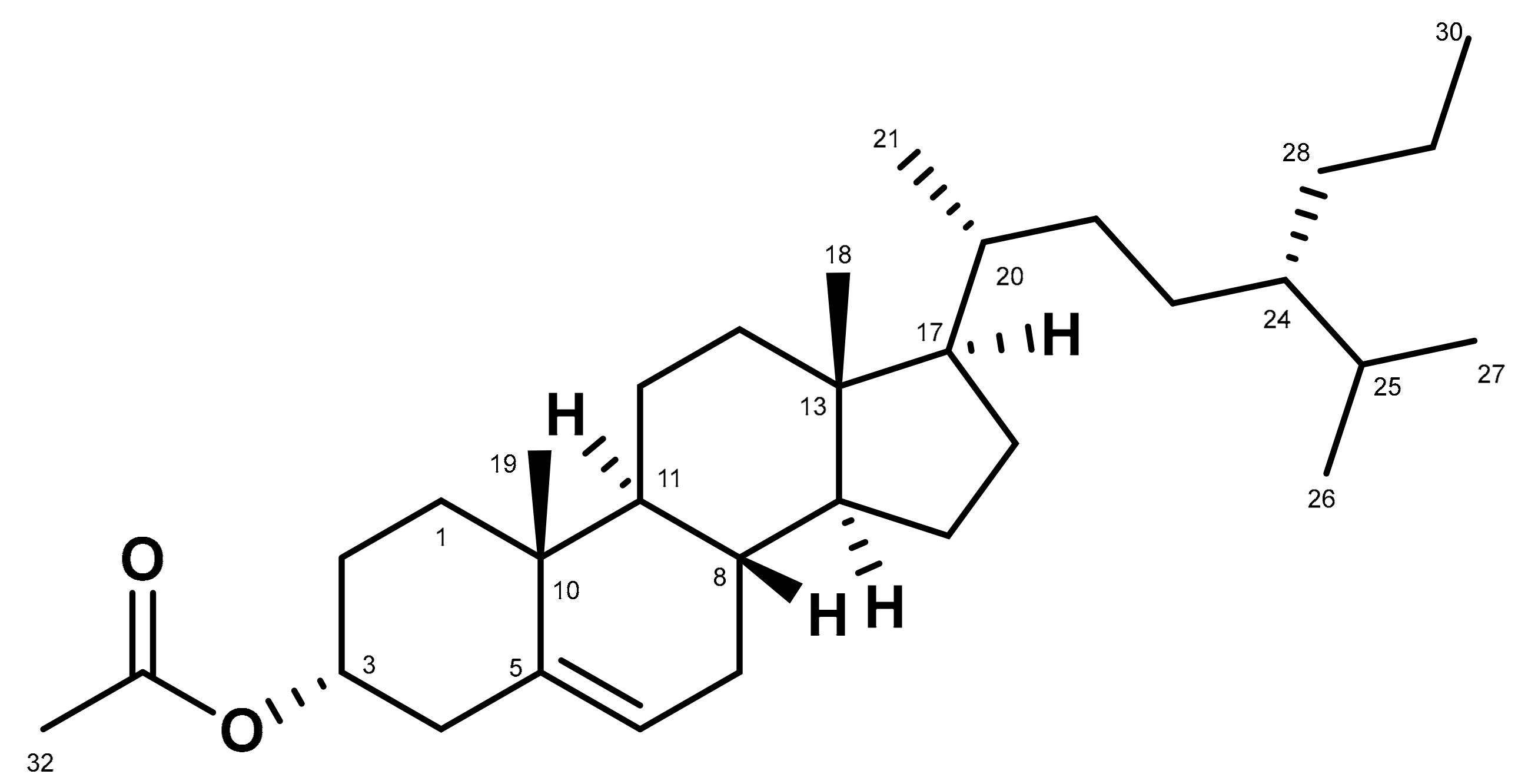
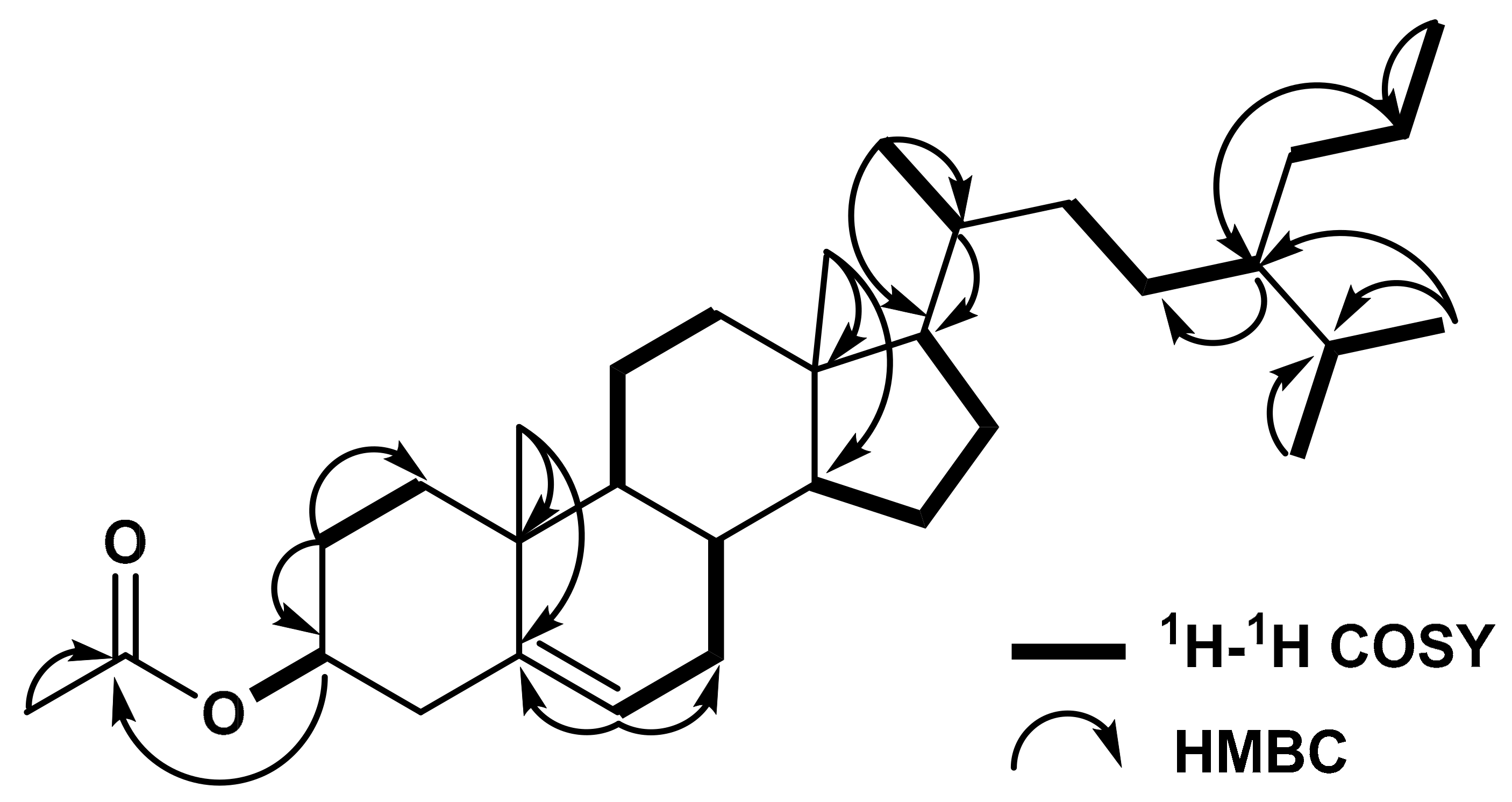
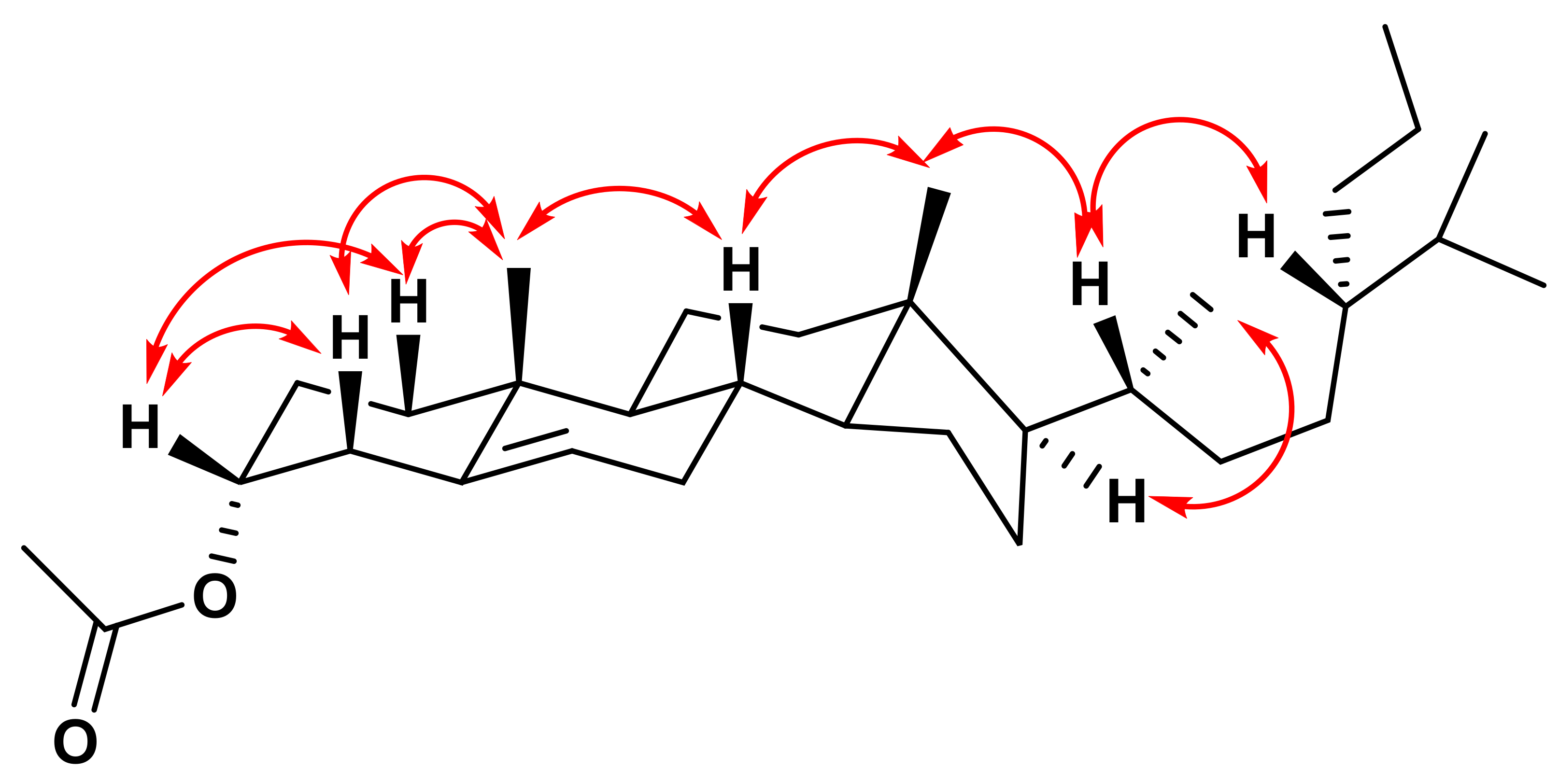
2. Results
Extraction and Isolation
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Cytotoxic Bioassay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mabberley, D.J.; Pannel, C.M.; Sing, A.M. Meliaceae. In Flora Malesiana; Series I. Spermatophyta; Rijksherbarium, Foundation Flora Malesiana: Leiden, The Netherlands, 1995; Volume 12, p. 407. [Google Scholar]
- Inada, A.; Murata, H.; Inatomi, Y.; Nakanishi, T.; Darnaedi, D. Pregnanes and triterpenoid hydroperoxides from the leaves of Aglaia grandis. Phytochemistry 1997, 45, 1225–1228. [Google Scholar] [CrossRef]
- Heyne, K. The Useful Indonesian Plants; Research and Development Agency, Ministry of Forestry: Jakarta, Indonesia, 1982; pp. 1029–1031. [Google Scholar]
- Nugroho, B.W.; Edrada, R.A.; Wray, V.; Witte, L.; Bringmann, G.; Gehling, M.; Proksch, P. An insectisidal rocaglamide derivates and related compounds from Aglaia odorata (Meliaceae). Phytochemistry 1999, 51, 367–376. [Google Scholar] [CrossRef]
- Harneti, D.; Tjokronegoro, R.; Safari, A.; Supratman, U.; Loong, X.; Mukthar, M.R.; Mohamad, K.; Awang, K.; Hayashi, H. Cytotoxic triterpenoid from the bark of Aglaia smithii. Phytochem. Lett. 2012, 5, 496–499. [Google Scholar] [CrossRef]
- Harneti, D.; Supriadin, A.; Ulfah, M.; Safari, A.; Supratman, U.; Awang, K.; Hayashi, H. Cytotoxic constituents from the bark of Aglaia eximia (Meliaceae). Phytochem. Lett. 2014, 8, 28–31. [Google Scholar] [CrossRef]
- Sianturi, J.; Harneti, D.; Darwati; Mayanti, T.; Supratman, U.; Awang, K. A New (–)-5′,6-dimethoxyisolariciresinol-(3″,4″-dimethoxy)-3α-O-β-D-glucopyranoside from the bark of Aglaia eximia (Meliaceae). Nat. Prod. Res. 2016, 30, 2204–2208. [Google Scholar] [CrossRef]
- Sianturi, J.; Purnamasari, M.; Darwati; Harneti, D.; Mayanti, T.; Supratman, U.; Awang, K.; Hayashi, H. New bisamide compounds from the bark of Aglaia eximia (Meliaceae). Phytochem. Lett. 2015, 13, 297–301. [Google Scholar] [CrossRef]
- Farabi, K.; Harneti, D.; Nurlelasari; Maharani, R.; Hidayat, A.T.; Awang, K.; Supratman, U.; Shiono, Y. New cytotoxic protolimonoids from the stem bark of Aglaia argentea (Meliaceae). Phytochem. Lett. 2017, 21, 211–215. [Google Scholar] [CrossRef]
- Farabi, K.; Harneti, D.; Nurlelasari; Maharani, R.; Hidayat, A.T.; Supratman, U.; Awang, K.; Shiono, Y. Cytotoxic Steroids from the Bark of Aglaia argentea (Meliaceae). Chiang Mai Univ. J. Nat. Sci. (CMU) 2017, 16, 293–306. [Google Scholar] [CrossRef]
- Farabi, K.; Harneti, D.; Nurlelasari; Maharani, R.; Hidayat, A.T.; Awang, K.; Supratman, U.; Shiono, Y. New cytotoxic pregnane-type steroid from the Stem Bark of Aglaia elliptica (Meliaceae). Rec. Nat. Prod. 2018, 12, 121–127. [Google Scholar] [CrossRef]
- Hidayat, A.T.; Farabi, K.; Harneti, D.; Maharani, R.; Darwati; Nurlelasari; Mayanti, T.; Setiawan, A.S.; Supratman, U.; Shiono, Y. Cytotoxicity and Structure Activity Relationship of Dammarane-Type Triterpenoids from the Bark of Aglaia elliptica against P-388 Murine Leukemia Cells. Nat. Prod. Sci. 2017, 23, 291–298. [Google Scholar] [CrossRef]
- Hua, L.; Qi, W.Y.; Husain, S.H.; Gao, K.; Arfan, M. Higly oxygenated stigmastane-type steroids from the aerial parts of Vernonia anthelmintica Willd. Steroids 2012, 77, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M.; Kokke, W.C.M.C.; Fenical, W.; Djerassi, C. Isolation of Two New C30 Sterols, (24E)-24-N-Propylidenecholesterol and 24ξ-N-Propylcholesterol, from a cultured Marine Chrysophyte. Steroids 1980, 35, 219–231. [Google Scholar] [CrossRef]
- Giner, J.-L.; Li, X.; Boyer, G.L. Sterol Composition of Aureumbra lagunensis, The Texas Brown Tide Alga. Phytochemistry 2001, 57, 787–789. [Google Scholar] [CrossRef]
- Giner, J.-L.; Li, X. Stereospecific synthesis of 24-Propylcholesterol Isolated from The Texas Brown Tide. Tetrahedron 2000, 56, 9575–9580. [Google Scholar] [CrossRef]
- Supriatno; Nurlelasari; Herlina, T.; Harneti, D.; Maharani, R.; Hidayat, A.T.; Mayanti, M.; Supratman, U.; Azmi, M.N.; Shiono, Y. A new limonoid from stembark of Chisocheton pentandrus (Meliaceae). Nat. Prod. Res. 2018, 32, 2610–2616. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, R.M. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Hadisaputri, Y.E.; Pharm, D.; Miyazaki, T.; Suzuki, S.; Yokobori, T.; Kobayashi, T.; Tanaka, N.; Inose, T.; Sohda, M.; Kuwano, H. TNFAIP8 overexpression: Clinical relevance to esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2012, 19, S589–S596. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutagaol, R.P.; Harneti, D.; Hidayat, A.T.; Nurlelasari, N.; Maharani, R.; Katja, D.G.; Supratman, U.; Awang, K.; Shiono, Y. (22E,24S)-24-Propylcholest-5en-3α-acetate: A New Steroid from the Stembark Aglaia angustifolia (Miq.) (Meliaceae). Molbank 2020, 2020, M1112. https://doi.org/10.3390/M1112
Hutagaol RP, Harneti D, Hidayat AT, Nurlelasari N, Maharani R, Katja DG, Supratman U, Awang K, Shiono Y. (22E,24S)-24-Propylcholest-5en-3α-acetate: A New Steroid from the Stembark Aglaia angustifolia (Miq.) (Meliaceae). Molbank. 2020; 2020(1):M1112. https://doi.org/10.3390/M1112
Chicago/Turabian StyleHutagaol, Ricson P., Desi Harneti, Ace T. Hidayat, Nurlelasari Nurlelasari, Rani Maharani, Dewa Gede Katja, Unang Supratman, Khalijah Awang, and Yoshihito Shiono. 2020. "(22E,24S)-24-Propylcholest-5en-3α-acetate: A New Steroid from the Stembark Aglaia angustifolia (Miq.) (Meliaceae)" Molbank 2020, no. 1: M1112. https://doi.org/10.3390/M1112
APA StyleHutagaol, R. P., Harneti, D., Hidayat, A. T., Nurlelasari, N., Maharani, R., Katja, D. G., Supratman, U., Awang, K., & Shiono, Y. (2020). (22E,24S)-24-Propylcholest-5en-3α-acetate: A New Steroid from the Stembark Aglaia angustifolia (Miq.) (Meliaceae). Molbank, 2020(1), M1112. https://doi.org/10.3390/M1112