Abstract
The bis(N′-(3-chlorobenzoyl)isonicotinohydrazide)iron(III) complex was synthesised from N′-(3-chlorobenzoyl)isonicotinohydrazide and iron(III) metal by reflux in an ethanol solution. The title compound was characterised by Fourier-transform infrared spectroscopy (FTIR) spectroscopy, differential thermal analysis/thermogravimetric analysis (DTA/TGA) and UV-visible spectroscopy. The results indicate that coordination of the iron(III) ion to the ligand increased its thermal stability.
1. Introduction
Hydrazide is an organic compound with a core hydrazine functional group and at least one hydrogen atom replaced by a substituent containing an acyl group. There is increasing interest among researchers in the development of hydrazide compounds, as they have been demonstrated to have a range of activities including antituberculosis [1], anticancer [2], analgesic, anti-inflammatory [3], anti-human immunodeficiency virus (HIV) [4], antidiabetic [5] and antimalarial [6] effects.
One of these hydrazide compounds is isoniazid. Isoniazid, also known as isonicotinylhydrazide (INH), is an antibiotic used for the treatment of tuberculosis. It has attracted much attention due to its demonstrated biological activity as an antituberculosis [7,8,9,10,11,12,13,14] and anticancer [15,16,17] agent.
In recent years, our research group has synthesised and tested isonicotinylhydrazide derivatives, namely N-benzoylisonicotinohydrazide. These compounds are known to have antituberculosis activity. One of the synthesised compounds was N′-(3-chlorobenzoyl) isonicotinohydrazide as a result of the reaction between isonicotinohydrazide with 2-Chlorobenzoyl chloride through an acylation reaction and this compound has been tested for activity in mycobacterium tuberculosis (H37Rv) with a Minimum Inhibitory Concentration value (25 ppm) [18,19]. One of the widely used methods for drug development is the modification of compounds into complexes with metals, as the formation of complex compounds has been proven to increase the pharmacological activity [14,16,20,21]. Recently, the bis (N′-(3-chlorobenzoyl) isonicotinohydrazide)iron(III) complex has been synthesised as an antituberculosis drug candidate.
2. Results and Discussion
Synthesis of the bis(N′-(3-chlorobenzoyl)isonicotinohydrazide)iron(III) complex was performed through a reaction to create coordinate covalent bonds between the Fe (III) ion and N′-(3-chloro benzoyl)isonicotinohydrazide compound. The solvent used in the synthesis of the complex was ethanol. This solvent was chosen because the compound N′-(3-chlorobenzoyl)isonicotinohydrazide has good solubility in ethanol.
Complex formation was achieved by reflux for 5 h at 75 °C while stirring using a magnetic stirrer. To form the complex, the N′-(3-chlorobenzoyl)isonicotinohydrazide (2 mmol) and FeCl3·6H2O (1 mmol) were used. The reflux method was chosen for the reaction to maintain stability so that complex synthesis reactions were successful, even in hot conditions, so that the reactant moles and products were maintained [10,22,23]. The reaction conditions of 75 °C and constant stirring (using a magnetic stirrer) aimed to accelerate and optimize the synthesis reaction process. Increasing the temperature and stirring the reaction mixture increases the kinetic energy for collision, therefore, the chances of a reaction are greater and the reaction progresses faster [24,25].
After the reflux was finished, the reflux product was evaporated in an electric water bath at 60 °C to allow the remaining solvent to evaporate. After that, the precipitate was roasted at 60 °C until a constant weight was obtained. The yield of the complex compound was 104 mg (43.91%). The synthesis obtained a brown powder that was odorless and soluble in hot ethanol.
For identification and characterization, the title compound was analyzed by UV-visible spectroscopy, Fourier-transform infrared spectroscopy (FTIR) spectroscopy, mass spectrometry and differential thermal analysis/thermogravimetric analysis (DTA/TGA).
During synthesis of the title complex, formation of the bis(N′-(3-chlorobenzoyl)isonicotinohydrazide)iron(III) complex was indicated by a shift in maximum wavelength (λmax) in the electronic spectrum to N′-(3-chlorobenzoyl)isonicotino hydrazide.
As shown in Figure 1, the λmax of N′-(3-chlorobenzoyl)isonicotinohydrazide was obtained at 268 nm and the λmax of the title complex was obtained at 265 nm. The formation of the title complex was indicated by weakening of the shift from 268 nm (Figure 1a) to 265 nm (Figure 1b).
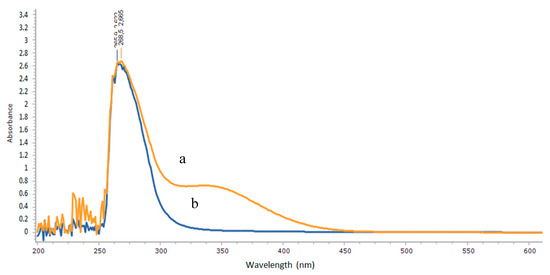
Figure 1.
Electronic spectra of N′-(3-chlorobenzoyl)isonicotinohydrazide (a) and the title complex (b) in dimethyl sulfoxide.
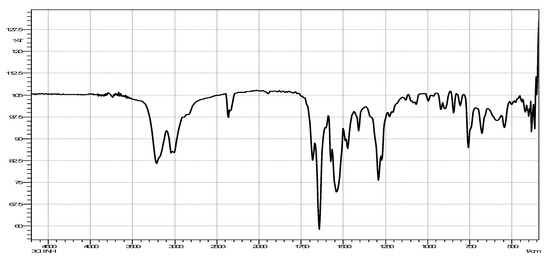
Figure 2.
Infrared (IR) spectrum of N′-(3-chlorobenzoyl)isonicotinohydrazide.
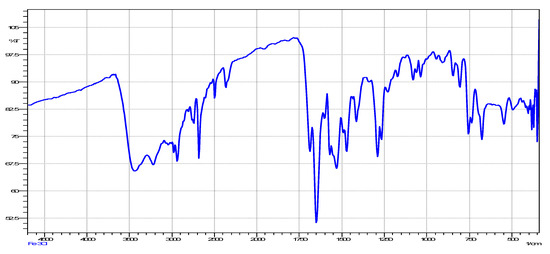
Figure 3.
Infrared spectrum of the title complex compound.
The IR spectrum for N′-(3-chlorobenzoyl)isonicotinohydrazide (Figure 2) showed a band at 1640 cm−1, representing carbonyl stretching. This band shifted to 1648 cm−1 in the complex (Figure 3), indicating that coordination to iron occurred with slight strengthening of the C=O bond. The υ(Fe-O) can be seen at 545 cm−1 and the υ (Fe-N) can be seen at 491 cm−1 [13,18].
The mass spectrum is presented in Figure 4. The molecular ion peak at m/z 609.0142, corresponding to the molecular weight of the complex, was detected in the mass spectrum of the title complex [Fe(C13H10ClN3O2)2].
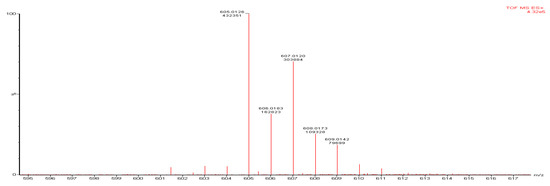
Figure 4.
Mass spectrum of the title complex compound [Fe(C13H10ClN3O2)2].
The results of DTA/TGA analysis of the title complex are presented in Figure 5. The decomposition process of the title complex was observed from 100 °C to 400 °C.
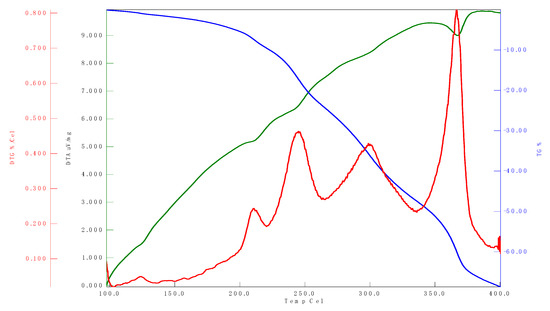
Figure 5.
Thermogravimetric curve of the title complex under N2 flow.
As shown in Figure 5, there is one endothermic peak at 383 °C. The thermogram of TG analysis showed the release of molecules marked by a reduction in mass at a certain temperature. The endothermic peak at 383 °C indicates that the decomposition complex begins with the release of several water molecules [10].
From the identification of IR spectrum in complex compound (Figure 3) and peaks that appear in the mass spectrum, it can be proposed the structure of the title complex as in Figure 6b [26].
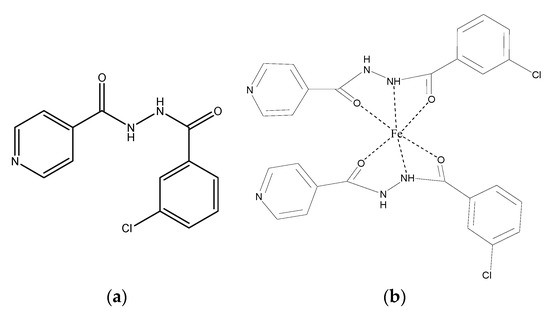
Figure 6.
The structure of N′-(3-chloro benzoyl)isonicotinohydrazide (a) and the title complex proposed (b).
3. Materials and Methods
3.1. Materials
All the chemicals were purchased from standard commercial suppliers. The chemicals used in this study included ethanol, dimethyl sulfoxide (DMSO), FeCl3·6H2O, nitrogen gas and KBr pellets. The electronic spectra were recorded in DMSO solution in the range 185–800 nm on a Shimadzu UV-3600 plus UV/VIS/NIR spectrophotometer (Shimadzu Corporation, Kyoto Prefecture, Japan). IR spectra were recorded in KBr on a Jasco FT-IR 5300 spectrometer (Jasco Inc., Mary’s Court Easton, MD, USA). MS spectra were recorded by high-resolution mass spectrometry (Waters Corp., Milford, MA, USA). Thermogravimetric analysis was performed using a STA 449 F1 Jupiter analyzer (NETZSCH, Selb, Germany) [27].
3.2. Synthesis of Bis(N′-(3-chlorobenzoyl)isonicotinohydrazide)iron(III) Complex
A solution of N′-(3-chlorobenzoyl)isonicotinohydrazide (192 mg, 0.699 mmol) in ethanol (25 mL) was slowly added to a solution of FeCl3·6H2O (105 mg, 0.39 mmol) in ethanol (15 mL). The mixture was stirred, then reflux was performed at 75 °C for about 7 h. It was then evaporated in a water bath at 60 °C. The resulting precipitate was oven-dried at 60 °C, then further dried in a vacuum desiccator.
Author Contributions
R.M. performed the synthesis and prepared the manuscript; Susanti assisted with characterisation; R.R. edited and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Penelitian Dosen Pemula (PDP) Grant from the Ministry of Research, Technology and Higher Education Indonesia in 2019.
Acknowledgments
The authors thank Perjuangan University and STIKes Bakti Tunas Husada Tasikmalaya for the provision of facilities to complete this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Metwally, K.A.; Abdel-Aziz, L.M.; Lashine, E.S.M.; Husseiny, M.I.; Badawy, R.H. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: Synthesis and preliminary evaluation as antimicrobial agents. Bioorg. Med. Chem. 2006, 14, 8675–8682. [Google Scholar] [CrossRef]
- Rodrigues, F.A.R.; Oliveira, A.C.A.; Cavalcanti, B.C.; Pessoa, C.; Pinheiro, A.C.; De Souza, M.V.N. Biological evaluation of isoniazid derivatives as an anticancer class. Sci. Pharm. 2014, 82, 21–28. [Google Scholar] [CrossRef]
- Nassiri Koopaei, M.; Javad Assarzadeh, M.; Almasirad, A.; Ghasemi-Niri, S.F.; Amini, M.; Kebriaeezadeh, A.; Koopaei, N.N.; Ghadimi, M.; Tabei, A. Synthesis and analgesic activity of novel hydrazide and hydrazine derivatives. Iran. J. Pharm. Res. 2013, 12, 721–727. [Google Scholar] [CrossRef]
- Al-Mawsawi, L.Q.; Dayam, R.; Taheri, L.; Witvrouw, M.; Debyser, Z.; Neamati, N. Discovery of novel non-cytotoxic salicylhydrazide containing HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6472–6475. [Google Scholar] [CrossRef]
- Smalley, T.L.; Peat, A.J.; Boucheron, J.A.; Dickerson, S.; Garrido, D.; Preugschat, F.; Schweiker, S.L.; Thomson, S.A.; Wang, T.Y. Synthesis and evaluation of novel heterocyclic inhibitors of GSK-3. Bioorg. Med. Chem. Lett. 2006, 16, 2091–2094. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.P.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N.; et al. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Sivasubramanian, V.K.; Ganesan, M.; Rajagopal, S.; Ramaraj, R. Iron(III)-salen complexes as enzyme models: Mechanistic study of oxo(salen)iron complexes oxygenation of organic sulfides. J. Org. Chem. 2002, 67, 1506–1514. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef]
- Ogunniran, K.O.; Adekoya, J.A.; Eromosele, C.O.E.; Siyanbda, T.O.; Kayode, A.; Mesubi, M.A.; Narender, T. Transition metal complexes of (E)-N′-(4-cyanobenzylidene)nicotinohydrazide): Synthesis, structural and anti-mycobacterial activity study. J. Appl. Sci. 2015, 15, 1210–1222. [Google Scholar] [CrossRef][Green Version]
- Jawoor, S.S.; Patil, S.A.; Toragalmath, S.S. Synthesis and characterization of heteroleptic Schiff base transition metal complexes: A study of anticancer, antimicrobial, DNA cleavage and anti-TB activity. J. Coord. Chem. 2018, 71, 271–283. [Google Scholar] [CrossRef]
- Arayne, S.; Sultana, N.; Haroon, U.; Mesaik, M.A. Synthesis, characterization, antibacterial and anti-inflammatory activities of enoxacin metal complexes. Bioinorg. Chem. Appl. 2009, 2009, 914105. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.D.; Dixit, S.R.; Gadag, S.; Kulkarni, V.H.; Aminabhavi, T.M. Molecular docking, synthesis, and antimycobacterial activities of pyrrolyl hydrazones and their copper complexes. Res. Rep. Med. Chem. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Hamdani, H.E.L.; Amane, M.E.L.; Atmani, Z.; Haddad, M. Synthesis, characterization and biological activities of cis-trans complexes [M(phen)(caf)2X2]M = Co(II), Fe(II), Mn(II), Cu(II); X: SCN−, CN−; caf: Caffeine; phen: (1,10)phenanthroline. J. Mater. Environ. Sci. 2016, 7, 3100–3109. [Google Scholar]
- Ramadevi, P.; Singh, R.; Prajapati, A.; Gupta, S.; Chakraborty, D. Cu(II) complexes of isoniazid Schiff bases: DNA/BSA binding and cytotoxicity studies on A549 cell line. Adv. Chem. 2014, 2014, 630575. [Google Scholar] [CrossRef]
- Hassan, S.; Channar, P.A.; Larik, F.A.; Saeed, A.; Shah, H.S.; Lecka, J.; Iqbal, J. Synthesis of novel (E)-1-(2-(2-(4(dimethylamino) benzylidene) hydrazinyl)-4-methylthiazol-5-yl)ethanone derivatives as ecto-5′-nucleotidase inhibitors. R. Soc. Open Sci. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Firmino, G.S.S.; de Souza, M.V.N.; Pessoa, C.; Lourenco, M.C.S.; Resende, J.A.L.C.; Lessa, J.A. Synthesis and evaluation of copper(II) complexes with isoniazid-derived hydrazones as anticancer and antitubercular agents. BioMetals 2016, 29, 953–963. [Google Scholar] [CrossRef]
- Hegde, D.; Naik, G.N.; Vadavi, R.S.; Shyam Kumar, V.; Barretto, D.A.; Gudasi, K.B. Transition metal complexes of N′-(2-(hydroxyimino)propanoyl)isonicotinohydrazide: Synthesis, characterization, DNA interaction and anticancer evaluation. Inorg. Chim. Acta 2017, 461. [Google Scholar] [CrossRef]
- Ruswanto, R.; Mardianingrum, R.; Lestari, T.; Nofianti, T.; Rahayuningsih, N. Synthesis and molecular docking of isonicotinohydrazide derivatives as anti-tuberculosis candidates. Mal. J. Fund. Appl. Sci. 2019, 15, 367–371. [Google Scholar] [CrossRef]
- Mardianingrum, R.; Ruswanto, R.; Nurjanah, R.; Nofianti, T.; Apriliani, A.Y. The biology activities of isonicotinohydrazide derivatives as an anti-tuberculosis candidate. J. Phys. Conf. Ser. 2019, 1179, 012181. [Google Scholar] [CrossRef]
- Baile, M.B.; Kolhe, N.S.; Deotarse, P.P.; Jain, A.S.; Kulkarni, A.A. Metal Ion Complex-Potential Anticancer Drug—A Review. Int. J. Pharma Res. Rev. 2015, 4, 59–66. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Ningtyas, A.P.; Martak, F. Sintesis dan uji toksisitas kompleks kobalt(II) dengan ligan (6E)-(N2)-((E)-2-(6-aminopiridin-2-ilimino)-1,2-difeniletilidin)piridin-2,6-diamina. Jurnal Sains Dan Seni ITS 2016, 5, 3–7. [Google Scholar]
- Lin, P.Z.; Chun, L.C.; Zhou, J.; Ming, X.L.; Yan, J.W. Synthesis, crystal structure and antitumor study of an iron(III) complex of 2-acetylpyrazine N(4)-methylthiosemicarbazone. Z. Naturforsch. B Chem. Sci. 2008, 63, 1257–1261. [Google Scholar]
- Dharmayanti, A.; Martak, F. Sintesis senyawa aktif kompleks mangan(II) dengan ligan 2(4-nitrofenil)-4,5-difenil-1H-imidazol. Jurnal Sains Dan Seni ITS 2015, 4, 52–56. [Google Scholar]
- Goeswin, A. Sediaan Farmasi Padat, Printed; ITB Publisher: Bandung, Indonesia, 2013; pp. 145–147. [Google Scholar]
- Rue, E.L.; Brulan, K.W. Complexation of iron(II1) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar. Chem. 1995, 50, 117–138. [Google Scholar] [CrossRef]
- Ruswanto, R.; Sarwatiningsih, Y.; Pratita, A.T.K.; Dewi, R. Synthesis and characterization of Fe(III) complex with N′-(3-nitrobenzoyl) isonicotinohydrazide as an anti-tuberculosis candidate. J. Phys. Conf. Ser. 2019, 1179, 012136. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).