Abstract
The X-ray structure of the title compound contains eight molecules in the unit cell which form the basis of a herringbone arrangement of hydrogen bonded ribbons.
1. Introduction
The simple heterocyclic compound tetrahydro-1,4-thiazine-3,5-dione (or thiomorpholine-3,5-dione) 1, the cyclic imide of thiodiglycolic acid 2, was first prepared in 1948 [1], and IR and NMR spectroscopic data were reported in 2001 [2]. Following our recent report of the first generation of the parent 1,4-oxazine [3], we were interested to explore routes to the analogous 1,4-thiazine and many of these attempts have involved starting from 1, although we have so far been unable to reproduce the reported [1] direct reduction of 1 to 1,4-thiazine over aluminium powder. In the course of these studies, compound 1 has been prepared in large quantity and we report here the molecular and crystal structure of 1 as determined by X-ray diffraction. The observed crystal structure is discussed in comparison with those in similar previously reported structures.
2. Results
Compound 1 was readily prepared using the literature method [1] involving heating commercially available thiodiglycolic acid 2 with an excess of aqueous ammonia and distilling off the water, first at atmospheric pressure and finally under vacuum (Scheme 1). The residue was subjected to kugelrohr distillation to give the product as colourless crystals which were directly suitable for the X-ray structure determination. Both IR and NMR (CD3SOCD3) spectra were in agreement with previously reported values [2], but we have also recorded 1H- and 13C-NMR data in CDCl3 (see Experimental Section and Supplementary Material).
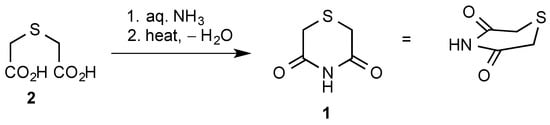
Scheme 1.
Synthetic route to 1 [1].
In the X-ray structure there are two distinct molecules (Figure 1, Table 1 and Table 2) both of which show an “envelope” structure with the sulfur atom above the plane containing the imide function and two CH2 groups. The molecular dimensions are similar but slightly different which reflects the different role of the two molecules in the hydrogen-bonding pattern (each molecule is hydrogen-bonded to two of the opposite type). In the first molecule the sulfur atom is 0.865 Å above the mean plane formed by C(2), C(3), N(4), C(5) and C(6) and the plane containing C(2), S(1) and C(6) is at an angle of 46.27° to the first plane. For the second molecule the corresponding parameters are 0.897 Å and 48.15°. In the unit cell there are eight molecules, four of each type, and these are arranged in a “herringbone” pattern when viewed along the a axis (Figure 2).
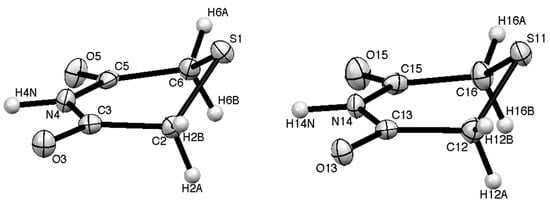
Figure 1.
The two distinct molecules in the molecular structure of 1 with numbering scheme.

Table 1.
Selected bond lengths.

Table 2.
Selected angles.
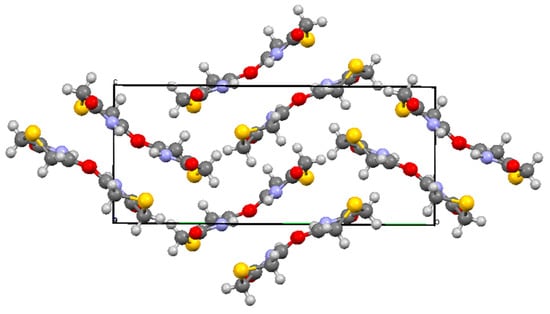
Figure 2.
Unit cell of 1 viewed along the a axis showing the 8 molecules present along with their hydrogen bonded partners.
The key feature of the overall structure is that each of the eight molecules in the unit cell forms the basis of a hydrogen-bonded ribbon (Figure 3) in which each molecule has its NH hydrogen-bonded to CO of an adjacent molecule and only one of its CO groups hydrogen-bonded to NH of a neighbour on the other side. The parameters (Table 3) show two strong and closely similar hydrogen-bonding interactions.
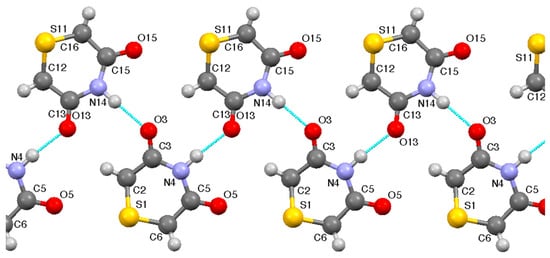
Figure 3.
Hydrogen bonded ribbon in the structure of 1.

Table 3.
Hydrogen bonding parameters for 1 (Å, °).
The only previous examples of this heterocyclic ring system to have their X-ray structures reported are a series of N-aryl compounds 3–9 (Figure 4) in which there is no opportunity for hydrogen-bonding and which adopt a range of different structures driven by molecular stacking interactions [4]. Perhaps the closest literature comparison is with the lactam thiomorpholine-3-one 10, in which a similar hydrogen-bonding interaction leads to simple helical chains of molecules [5]. In the corresponding tetrahydro-1,4-oxazinedione series also, there have been very few previous X-ray studies with the N-aryl compound 11 the only example located [6], while the parent dione 12 with the different 2,5-arrangement of carbonyl groups has also been reported [7].
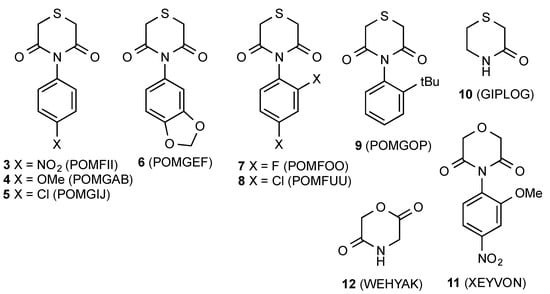
Figure 4.
Similar structures characterized by X-ray diffraction with CCDC Ref Codes.
In view of the interesting hydrogen-bonding pattern discovered for 1, it is clear that there is ample opportunity for further structural studies on such simple heterocyclic imides.
3. Experimental
Melting points were recorded on a Reichert hot-stage microscope (Reichert, Vienna, Austria) and are uncorrected. IR spectra were recorded on a Perkin-Elmer 1420 instrument (Perkin-Elmer, Waltham, MA, USA). NMR spectra were obtained for proton at 300 MHz and for carbon at 75 MHz using a Bruker AM300 instrument (Bruker, Billerica, MA, USA). Spectra were run at 25 °C on solutions in CD3SOCD3 with internal Me4Si as reference. Chemical shifts are reported in ppm to high frequency of the reference and coupling constants J are in Hz.
Tetrahydro-1,4-thiazine-3,5-dione (1)
Aqueous ammonia (d 0.88, 18 M, 15 mL, 270 mmol) was added cautiously to thiodiglycolic acid (15 g, 100 mmol) in a flask set up for distillation. The mixture was slowly heated and water distilled off first under atmospheric pressure and then at 20 Torr. The residue was then subjected to Kugelrohr distillation at 0.1 Torr to give the pure product (9.3 g, 71%) as colourless crystals; mp 118–120 °C (lit. mp 128 °C [1]); IR (nujol): 3420, 1728, 1270, 1194, 1143, 918, 856, 790, 657 cm−1; 1H-NMR (300 MHz, CD3SOCD3): δ 3.50 (s); 13C-NMR (75 MHz, CD3SOCD3): δ 170.5, 30.3; 1H-NMR (300 MHz, CDCl3): δ 8.56 (1H, br, NH), 3.48 (4H, s); 13C-NMR (75 MHz, CDCl3): δ 168.8, 30.9.
Crystal Data for C4H5NO2S, M = 131.15 g mol−1, colourless prism, crystal dimensions 0.12 × 0.12 × 0.12 mm, monoclinic, space group P21/n, a = 7.173(3), b = 18.603(6), c = 8.393(4) Å, β = 108.164(8)°, V = 1064.2(7) Å3, Z = 8, Dcalc = 1.637 g cm−3, T = 93 K, R1 = 0.0393, Rw2 = 0.0995 for 1661 reflections with I > 2σ(I) and 153 variables. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71075 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 1867063. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/getstructures. The structure was solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL, Version 2018/3, [8]). Hydrogen atoms were assigned riding isotropic displacement parameters and constrained to idealised geometries except for H(4N) and H(14N) which were refined with a distance restraint.
Supplementary Materials
The following are available online, Figure S1: 300 MHz 1H-NMR spectrum of 1 in CDCl3, Figure S2: 75 MHz 13C-NMR spectrum of 1 in CDCl3.
Author Contributions
P.-p.Y. prepared the compound; A.M.Z.S. collected the X-ray data and solved the structure; R.A.A. designed and performed the experiments, analysed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barkenbus, C.; Landis, P.S. The Preparation of 1,4-Thiazine. J. Am. Chem. Soc. 1948, 70, 684–685. [Google Scholar] [CrossRef]
- Aitken, R.A.; Farrell, D.M.M.; Kirton, E.H.M. Synthesis and Pyrolysis of Tetrahydro-1,4-oxazine-3,5-diones and Tetrahydro-1,4-thiazine-3,5-diones. Chem. Heterocycl. Compd. 2001, 37, 1526–1531. [Google Scholar] [CrossRef]
- Aitken, R.A.; Aitken, K.M.; Carruthers, P.G.; Jean, M.A.; Slawin, A.M.Z. 1,4-Oxazine. Chem. Commun. 2013, 49, 11367–11369. [Google Scholar] [CrossRef] [PubMed]
- Szawkalo, J.; Maurin, J.K.; Plucinski, F.; Czarnocki, Z. Synthesis and dynamic stereochemistry of 4-aryl-thiomorpholine-3,5-dione derivatives. J. Mol. Struct. 2015, 1079, 383–390. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Parthasarathy, R.; Tsoucaris, G. Structure of thiomorpholine-3-one: Comments on the geometry of monocoordinated metal complexes. Acta Crystallogr. Sect. C 1988, 44, 2016–2018. [Google Scholar] [CrossRef]
- Bhuiyan, M.D.H.; Jensen, P.; Turner, P.; Try, A.C. 4-(2-Methoxy-4-nitrophenyl)morpholine-3,5-dione. Acta Crystallogr. Sect. E 2007, 63, o1115–o1116. [Google Scholar] [CrossRef]
- Martínez-Palau, M.; Urpí, L.; Solans, X.; Puiggalí, J. Morpholine-2,5-dione. Acta Crystallogr. Sect. C 2006, 62, o262–o264. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).