Abstract
The new ligand 4′-(5-methylfuran-2-yl)-2,2′:6′,2″-terpyridine (1) was prepared in one step from 2-acetylpyridine and 5-methylfurfural. The latter is an aldehyde that can be readily obtained from biomass. The new terpyridine molecule was characterized by 1H and 13C-NMR spectroscopy as well as by elemental analyses and HR-MS. Owing to its chelating properties, this new terpyridine molecule was tested as a ligand in a metal-catalyzed reaction: The Ni-catalyzed dimerization of benzyl bromide.
1. Introduction
2,2′:6′,2″-Terpyridine molecules (tpy) are a class of heterocyclic compounds that possess three pyridine moieties (Figure 1). These molecules can form complexes with a broad range of metals owing to the chelate effect. Therefore, tpy and their complexes have been widely studied [1]. Terpyridines and their complexes find applications in various fields, such as photovoltaic devices [2], sensors [3], medicinal chemistry [4], and for the construction of MOFs [5] just to name a few.
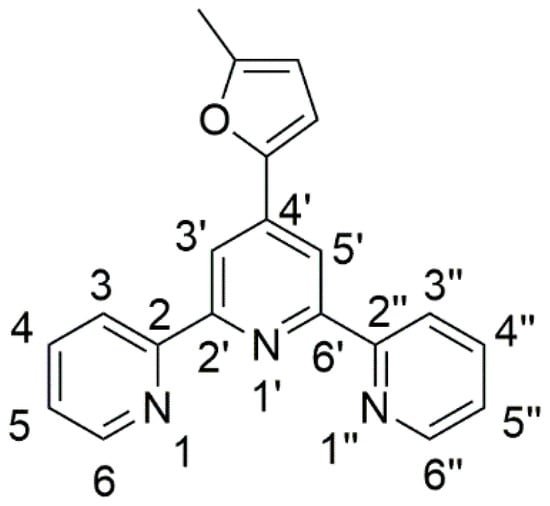
Figure 1.
Chemical structure and atom-numbering of 4′-(5-methylfuran-2-yl)-2,2′:6′,2″-terpyridine (1).
Many methods are available for the preparation of terpyridine derivatives [6,7,8]. A classical method, the so-called Kröhnke’s method [9,10] involves the reaction of 2-acetylpyridine and an aldehyde. Biomass-derived aldehyde furfural has been already used for the preparation of terpyridines [11]. Apart of providing terpyridines with interesting properties, the use of furfural in tpy preparation allows greener synthetic procedures since furfural is obtained from biomass and therefore renewable [12]. Furthermore, the use of furfural in the preparation of functionalized terpyridine derivatives allows the design of greener synthetic pathways [13].
5-Methylfurfural is another furan derivative that can be easily obtained from biomass [14]. To the best of our knowledge, this aldehyde has not been used for the preparation of a terpyridine molecule. This article describes the preparation of compound 1 (Figure 1) from 5-methylfurfural and its use as a ligand in metal-catalyzed reactions.
2. Results and Discussion
Terpyridine 1 was prepared by simply mixing 2-acetylpyridine and 5-methylfurfural in ethanol in the presence of potassium hydroxide and aqueous ammonia (Scheme 1), according to the method of Wang and Hanan [15].
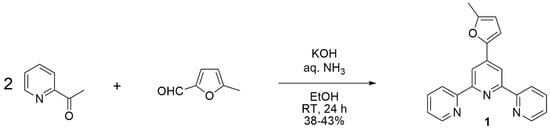
Scheme 1.
Preparation of terpyridine 1.
Since the product precipitates as a light yellow solid, it was easily isolated by filtration in 38% to 43% yield over three experiments. The synthetic protocol is very simple and provided a material that was sufficiently pure (>98% by quantitative 1H-NMR) to be used without purification. Nevertheless, an analytically pure sample was obtained by recrystallization in ethanol. The identity of the compound was confirmed by 1H and 13C-NMR spectroscopy, as well as by elemental analyses and HR-MS.
Terpyridines can be used as ligands in a vast list of metal-catalyzed reactions [16]. Thus compound 1 was tested in the nickel-catalyzed dimerization of alkyl halides [17,18]. In fact, it has been reported that 4,4′,4″-tritertbutyl-2,2′:6′,2″-terpyridine is an efficient ligand for this reaction [19]. Although 81% yield is obtained in the dimerization of benzyl bromide using 4,4′,4″-tritertbutyl-2,2′:6′,2″-terpyridine, substituting it by compound 1 resulted in a very low yield under the same conditions (Scheme 2, Table 1). This can be explained by the effects of the substituents onto the terpyridine, which can have important effects onto the outcome of reactions [20]. For instance, the nature of the substituents can influence the redox properties of the complex formed between the ligand and the metal [18] thus modifying the course of the reaction.
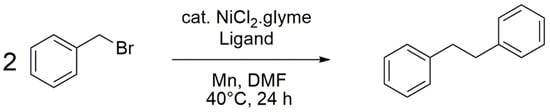
Scheme 2.
Ni-catalyzed dimerization of benzyl bromide.

Table 1.
Yields obtained for the Ni-catalyzed dimerization of benzyl bromide with 4,4′,4″-tritertbutyl-2,2′:6′,2″-terpyridine and compound 1 as ligands.
3. Materials and Methods
All reagents were purchased from commercial suppliers and used as received. Flash chromatography was carried out on a Combiflash Rf + Lumen (Teledyne ISCO, Lincoln, NE, USA) using Redisep Rf silica column (Teledyne ISCO, Lincoln, NE, USA). 1H and 13C-NMR spectra were recorded on a Brucker AC 400 (Bruker, Wissembourg, France) at 400 and 100 MHz, respectively using CDCl3 as a solvent. UV-Vis spectrum was recorded on a Cary 300 (Agilent Technologies, Santa Clara, CA, USA) using acetonitrile (C = 1.15 × 10−4 M) as solvent. Melting point was recorded with a Stuart SMP 10 melting point apparatus (Bibby Sterilin, Stone, UK) and is uncorrected. Elemental analysis was performed at Service d’Analyses Elementaires, UMR 7565 CNRS, Vandoeuvre-les-Nancy, France. HR-MS was recorded at Welience, Dijon, France.
3.1. Preparation of 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine
4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine (1): To a solution of 2-acetylpyridine (4.84 g; 40 mmol) in ethanol (100 mL) are added 5-methylfurfural (2.20 g; 20 mmol), 85% potassium hydroxide pellets (3.08 g; 47 mmol) and 25% aqueous ammonia (58 mL). The reaction mixture was stirred at room temperature for 24 h. The solid was then filtered on a glass-sintered funnel and washed with ice-cold 50% ethanol until washings were colorless. The product was dried under vacuum over phosphorus pentoxide. Compound 1 was obtained as a light yellow solid (2.42 to 2.71 g; 38% to 43%). An analytical sample was obtained by recrystallization in ethanol. Mp = 174 °C. 1H-NMR (CDCl3, 400 MHz), δ (ppm): 8.75 (ddd, 2H, H6, 6″, J = 4.8 Hz, J = 1.6 Hz, J = 0.8 Hz), 8.67 (s, 2H, H3′, 5′), 8.65 (d, 2H, H3, 3″, J = 8.0 Hz), 7.87 (dt, 2H, H4, 4″, J = 7.7 Hz, J = 1.8 Hz), 7.35 (ddd, 2H, H5, 5″, J = 7.4 Hz, J = 4.8 Hz, J = 1.1 Hz), 7.03 (d, 1H, H2-furyl, J = 3.3 Hz), 6.17 (dd, 1H, H3-furyl, J = 3.3 Hz, J = 0.9 Hz), 2.44 (s, 3H, CH3-furyl). 13C-NMR (CDCl3, 100 MHz), δ (ppm): 156.2, 155.7, 154.0, 150.2, 149.0, 139.7, 136.8, 123.7, 121.3, 114.5, 110.4, 108.4, 13.9. Elemental analysis for C20H15N3O: C, 76.66; H, 4.82; N, 13.41. Found C, 77.00; H, 4.93; N, 13.50. HR-MS: calc. for [C20H15N3O + H]+ 314.12773, found 314.12879. UV-Vis (nm, L·cm−1·mol−1): λabs = 229, ε = 24017; λabs = 251, ε = 21434; λabs = 286, ε = 33678; λabs = 312, ε = 28008.
3.2. Nickel-Catalyzed Dimerization of Benzyl Bromide with Compound 1 as a Ligand
In a test tube were successively added NiCl2·glyme (2.6 mg; 0.01 mmol), compound 1 (3.1 mg; 0.01 mmol), benzyl bromide (238 μL; 2.00 mmol), manganese powder (110.0 mg; 2.00 mmol), and DMF (2 mL). The test tube was stoppered and the mixture was stirred at 40 °C for 24 h. After cooling to room temperature, the crude solution was directly injected onto a 12 g-silica column and the product was purified by flash chromatography using hexane as eluent. The pure product was obtained as a white solid (3.6 mg; 2%). Analytical data match those reported in the literature [19,21].
4. Conclusions
A new member of the terpyridine family has been prepared and characterized. It was prepared from the biomass-derived reagent 5-methylfurfural. Its preparation was easy on the gram-scale. This new terpyridine was assessed for its potential application as a ligand in metal-catalyzed reactions. Although it was not efficient in the nickel-catalyzed dimerization of benzyl bromide, considering the vast number of reactions employing terpyridines as a ligand, 4′-(5-methylfuran-2-yl)-2,2′:6′,2″-terpyridine could be a promising tool in synthetic organic chemistry. Future work will focus on screening other reactions, in which this new terpyridine could be used.
Supplementary Materials
The following are available online, 1H and 13C-NMR, HR-MS spectra and UV-Vis spectra.
Author Contributions
J.H. conceived and carried out the experiments, analyzed data and prepared the manuscript. L.G. analyzed data and contributed to manuscript preparation.
Funding
This research was funded by Université de Franche-Comté (CHRYSALIDE Project).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Naidji, B.; Husson, J.; Et Taouil, A.; Brunol, E.; Sanchez, J.-B.; Berger, F.; Rauch, J.-Y.; Guyard, L. Terpyridine-based metallopolymer thin films as active layer in ammonia sensor device. Synth. Met. 2016, 221, 214–219. [Google Scholar] [CrossRef]
- Cummings, S.D. Platinum complexes of terpyridine: interaction and reactivity with biomolecules. Coord. Chem. Rev. 2009, 253, 1495–1516. [Google Scholar] [CrossRef]
- Young, D.C.; Yang, H.; Telfer, S.G.; Kruger, P.E. An Isoreticular Series of Zinc(II) Metal-Organic Frameworks Derived from Terpyridylcarboxylate Ligands. Inorg. Chem. 2017, 56, 12224–12231. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2’:6’,2’’-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2’:6’,2’’-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2’:6’,2’’-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1, 1–24. [Google Scholar] [CrossRef]
- Sasaki, I. Recent Uses of Kröhnke Methodology: A Short Survey. Synthesis 2016, 48, 1974–1992. [Google Scholar] [CrossRef]
- Husson, J.; Knorr, M. Syntheses and applications of furanyl-functionalised 2,2′:6′,2′′-terpyridines. Beilstein J. Org. Chem. 2012, 8, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; Ei Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4’,4’’-tricarboxy-2,2’:6’,2’’-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Yang, W.; Sen, A. Direct Catalytic Synthesis of 5-Methylfurfural from Biomass-Derived Carbohydrates. ChemSusChem 2011, 4, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4’-Aryl-2,2’:6’,2’’-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Winter, A.; Newkome, G.R.; Schubert, U.S. Catalytic Applications of Terpyridines and their Transition Metal Complexes. ChemCatChem 2011, 3, 1384–1406. [Google Scholar] [CrossRef]
- Anderson, T.J.; Jones, G.D.; Vicic, D.A. Evidence for a NiI Active Species in the Catalytic Cross-Coupling of Alkyl Electrophiles. J. Am. Chem. Soc. 2004, 126, 8100–8101. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Martin, J.L.; McFarland, C.; Allen, O.R.; Hall, R.E.; Haley, A.D.; Brandon, R.J.; Konovalova, T.; Desrochers, P.J.; Pulay, P.; Vicic, D.A. Ligand Redox Effects in the Synthesis, Electronic Structure, and reactivity of an Alkyl-Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc. 2006, 128, 13175–13183. [Google Scholar] [CrossRef] [PubMed]
- Prinsell, M.R.; Everson, D.A.; Weix, D.J. Nickel-catalyzed, sodium iodide-promoted reductive dimerization of alkyl halides, alkyl pseudohalides, and allylic acetates. Chem. Commun. 2010, 46, 5743–5745. [Google Scholar] [CrossRef] [PubMed]
- Huihui, K.M.M.; Shrestha, R.; Weix, D.J. Nickel-Catalyzed Reductive Conjugate Addition of Primary Alkyl Bromides to Enones To Form Silyl Enol Ethers. Org. Lett. 2017, 19, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Felpin, F.-X.; Fouquet, E. A Useful, Reliable and Safer Protocol for Hydrogenation and the Hydrogenolysis of O-Benzyl Groups: The In Situ Preparation of an Active Pd0/C Catalyst with Well-Defined Properties. Chem. Eur. J. 2010, 16, 12440–12445. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).