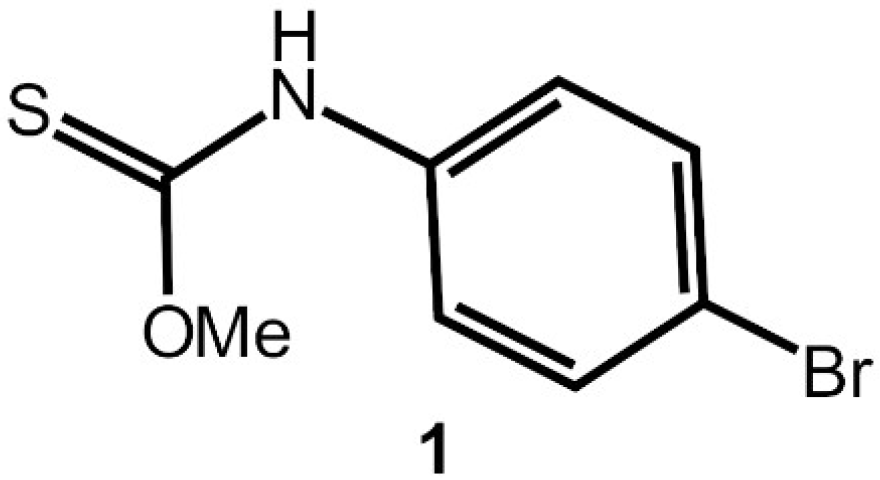
N-(4-Bromophenyl)methoxycarbothioamide
Abstract
1. Introduction
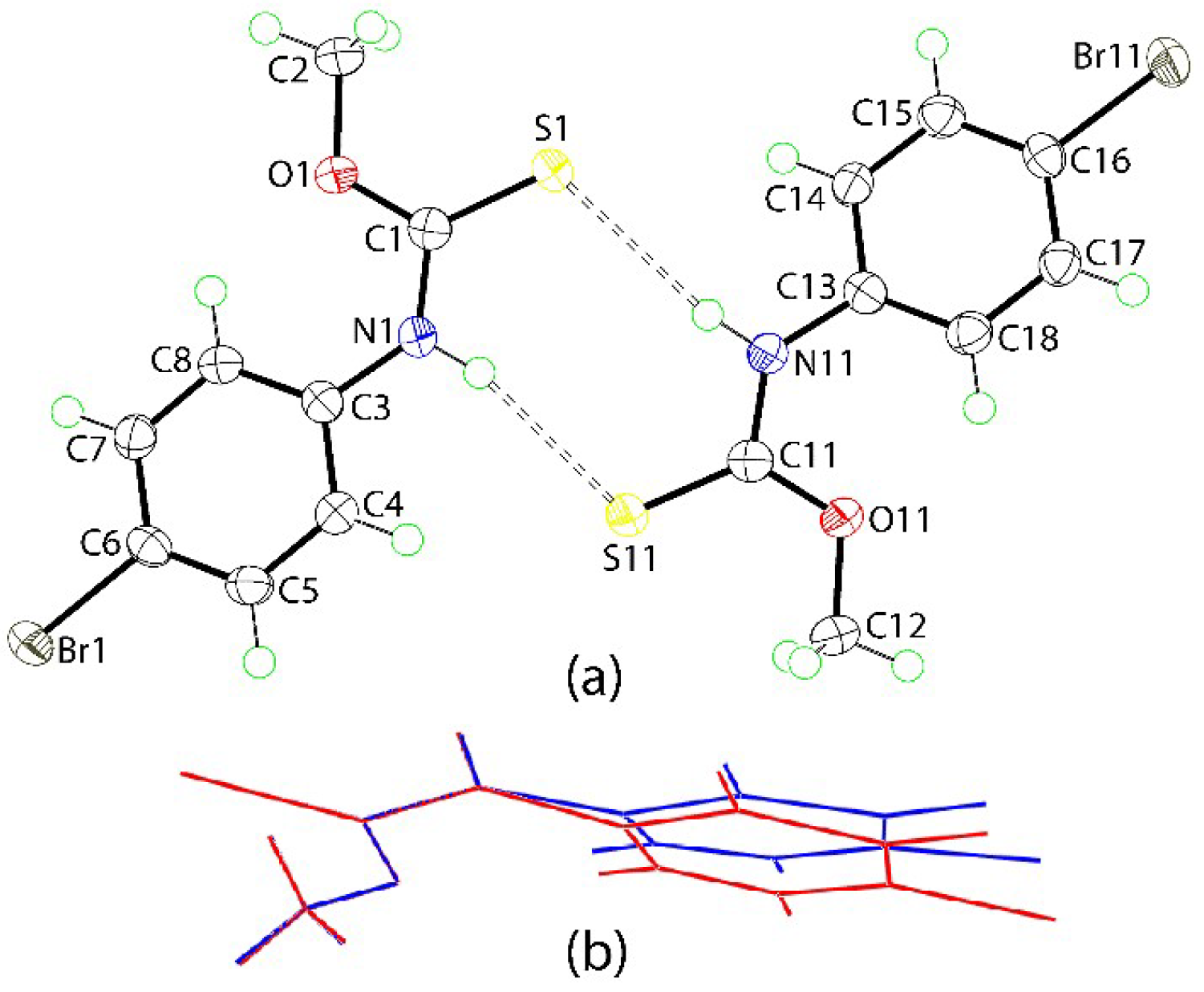
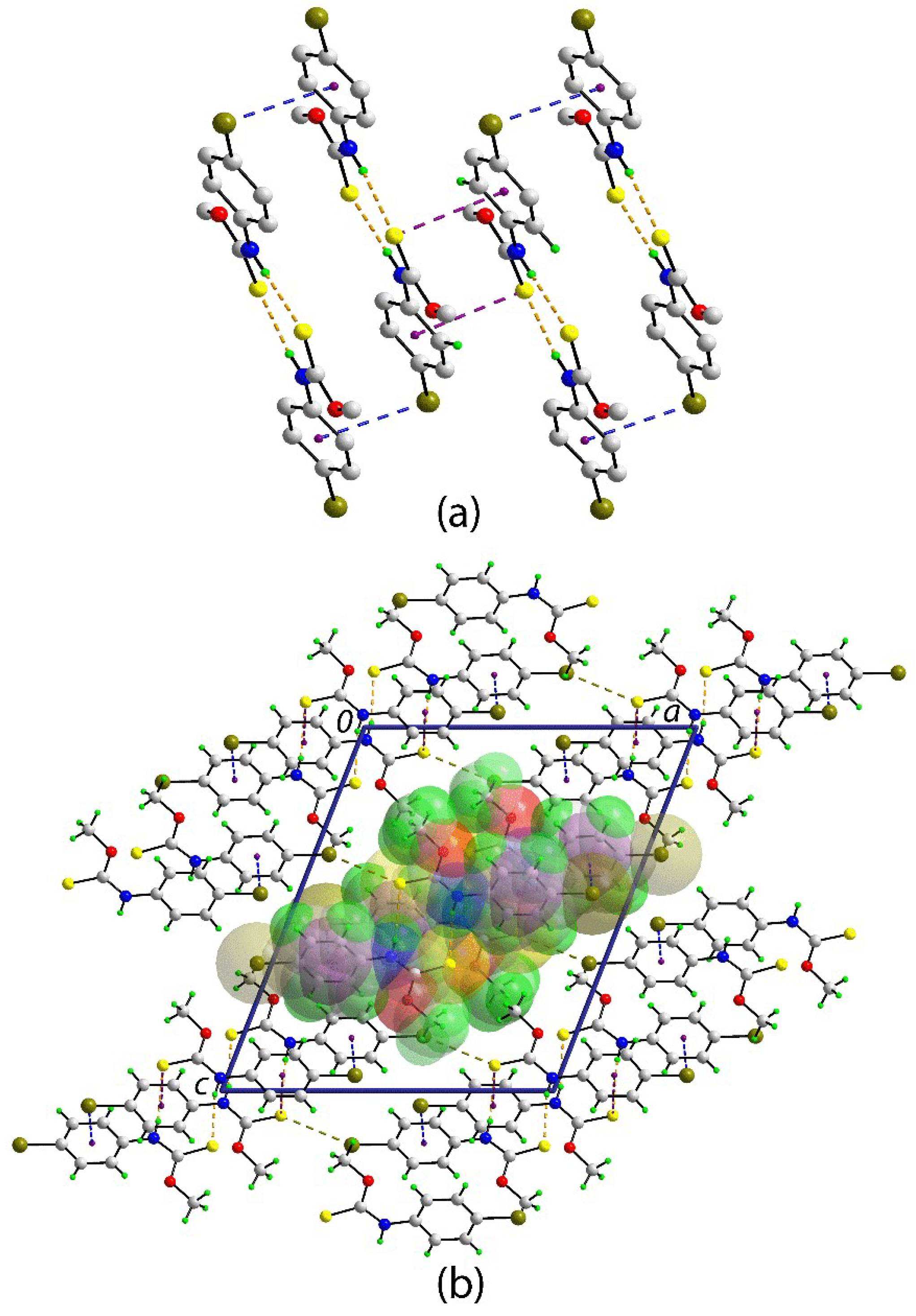
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterisation
3.3. Crystallography
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ooi, K.K.; Yeo, C.I.; Mahandaran, T.; Ang, K.P.; Akim, A.M.; Cheah, Y.K.; Seng, H.L.; Tiekink, E.R.T. G2/M cell cycle arrest on HT-29 cancer cells and toxicity assessment of triphenylphosphanegold(I) carbonimidothioates, Ph3PAu[SC(OR)=NPh], R = Me, Et, and iPr, during zebrafish development. J. Inorg. Biochem. 2017, 166, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Sim, J.H.; Khoo, C.H.; Goh, Z.J.; Ang, K.P.; Cheah, Y.K.; Fairuz, Z.A.; Halim, S.N.B.A.; Ng, S.W.; Seng, H.L.; et al. Pathogenic Gram-positive bacteria are highly sensitive to triphenylphosphanegold(O-alkylthiocarbamates), Ph3PAu[SC(OR)=N(p-tolyl)] (R = Me, Et and iPr). Gold Bull. 2013, 46, 145–152. [Google Scholar] [CrossRef]
- Ho, S.Y.; Cheng, E.C.C.; Tiekink, E.R.T.; Yam, V.W.W. Luminescent phosphine gold(I) thiolates: Correlation between crystal structure and photoluminescent properties in [R3PAu{SC(OMe)=NC6H4NO2-4}] (R = Et, Cy, Ph) and [(Ph2P-R-PPh2){AuSC(OMe)=NC6H4NO2-4}2] (R = CH2, (CH2)2, (CH2)3, (CH2)4, Fc). Inorg. Chem. 2006, 45, 8165–8174. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Khoo, C.H.; Chu, W.C.; Chen, B.J.; Chu, P.L.; Sim, J.H.; Cheah, Y.K.; Ahmad, J.; Halim, S.N.A.; Seng, H.L.; et al. The importance of Au…π(aryl) interactions in the formation of spherical aggregates in binuclear phosphane gold(I) complexes of a bipodal thiocarbamate dianion: A combined crystallographic and computational study, and anti-microbial activity. RSC Adv. 2015, 52, 41401–41411. [Google Scholar] [CrossRef]
- Ho, S.Y.; Bettens, R.P.A.; Dakternieks, D.; Duthie, A.; Tiekink, E.R.T. Prevalence of the thioamide {...H‒N‒C=S}2 synthon—solid-state (X-ray crystallography), solution (NMR) and gas-phase (theoretical) structures of O-methyl-N-aryl-thiocarbamides. CrystEngComm 2005, 7, 682–689. [Google Scholar] [CrossRef]
- Kuan, F.S.; Mohr, F.; Tadbuppa, P.P.; Tiekink, E.R.T. Principles of crystal packing in O-isopropyl-N-aryl-thiocarbamides: iPrOC(=S)N(H)C6H4-4-Y: Y = H, Cl, and Me. CrystEngComm 2007, 9, 574–581. [Google Scholar] [CrossRef]
- Jotani, M.M.; Yeo, C.I.; Tiekink, E.R.T. A new monoclinic polymorph of N-(3-methylphenyl)ethoxycarbothioamide: Crystal structure and Hirshfeld surface analysis. Acta Crystallogr. E 2017, 73, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.F.; Parken, E.R. 413. The unsaturation and tautomeric mobility of heterocyclic compounds. Part VI. The mobility of 5-substituted 1-hydroxybenzthiazoles, and the ultra-violet absorption of mobile and static derivatives of 1-hydroxybenzthiazole. J. Chem. Soc. 1935, 1755–1761. [Google Scholar] [CrossRef]
- Goeckeritz, D.; Pohloudek-Fabini, R. Halo and thiocyanato thiosemicarbazides and thiosemicarbazones and their behavior in paper chromatography. II. Pharmazie 1962, 17, 679–685. [Google Scholar]
- Goeckeritz, D.; Pohloudek-Fabini, R. Halo and thiocyanato thiourethans and their application to the determination of aliphatic alcohols by paper chromatography. Pharm. Zent. 1963, 102, 685–689. [Google Scholar]
- Bauman, R.A. Thioncarbamate esters. U.S. Patent US 3592835 A 19710713, 1971. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Jones, W.; Motherwell, W.D.S.; Shields, G.P. Crystal engineering and chloro-methyl interchange—A CSD analysis. Mol. Cryst. Liq. Cryst. 2001, 356, 337–353. [Google Scholar] [CrossRef]
- Ho, S.Y.; Lai, C.S.; Tiekink, E.R.T. O-Methyl N-phenylthiocarbamate. Acta Crystallogr. E 2003, 59, o1155–o1156. [Google Scholar] [CrossRef]
- Ho, S.Y.; Lai, C.S.; Tiekink, E.R.T. (E)-O-Methyl N-(4-methylphenyl)thiocarbamate. Acta Crystallogr. E 2007, 63, o1723–o1724. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO; Agilent Technologies Inc.: Santa Clara, CA, USA, 2015. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
Sample Availability: Samples of the compound is not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, C.I.; Tiekink, E.R.T. N-(4-Bromophenyl)methoxycarbothioamide. Molbank 2018, 2018, M1012. https://doi.org/10.3390/M1012
Yeo CI, Tiekink ERT. N-(4-Bromophenyl)methoxycarbothioamide. Molbank. 2018; 2018(3):M1012. https://doi.org/10.3390/M1012
Chicago/Turabian StyleYeo, Chien Ing, and Edward R.T. Tiekink. 2018. "N-(4-Bromophenyl)methoxycarbothioamide" Molbank 2018, no. 3: M1012. https://doi.org/10.3390/M1012
APA StyleYeo, C. I., & Tiekink, E. R. T. (2018). N-(4-Bromophenyl)methoxycarbothioamide. Molbank, 2018(3), M1012. https://doi.org/10.3390/M1012





